Protein targeted degradation chimera is an emerging protein degradation strategy that has been booming in recent years. It was first proposed by Craig Crews et al. in 2001. Its basic principle is to use bi-functional small molecules to induce target degradation through the ubiquitin-proteasome system. Protein ubiquitination, thereby achieving target protein degradation. Since ARV-110, the first protein targeted degradation chimera degrader targeting the androgen receptor, entered clinical research in 2019, the protein targeted degradation chimera field has entered a period of rapid development.
The BTK protein (Bruton’s tyrosine kinase), a tyrosine kinase and key regulator of the B-cell receptor (BCR) signaling pathway, is overactivated in B-cell lymphoma cells. Previous research by Rao Jue’s team showed that protein targeted degradation chimera technology can achieve effective degradation of wild-type and mutant BTK proteins. Since then, they have also developed a new generation of BTK degrader L18I, which shows better solubility and efficiency in degrading BTK.
Notably, in addition to B-cell lymphomas, BTK dysfunction also plays an important role in autoimmune diseases. In recent years, BTK inhibitors have been used for the treatment of autoimmune diseases, but due to issues with clinical efficacy and safety, they have experienced both success and failure in clinical trials. Although protein targeted degradation chimera-targeted degradation of BTK exhibits minimal off-target effects, no studies have explored the use of protein targeted degradation chimera degradation of BTK to treat autoimmune diseases.
On August 6, 2024, Liu Wanli and Rao Xuan of Tsinghua University and Ding Ning of Peking University Cancer Hospital published a research paper titled “PROTAC for Bruton’s tyrosine kinase degradation alleviates inflammation in autoimmune diseases” in the Cell Discovery journal.
This study shows that the protein targeted degradation chimera degrader L18I, which targets BTK (Bruton’s tyrosine kinase), can effectively treat the autoimmune disease lupus and its severe complication diffuse alveolar hemorrhage (DAH). L18I may become a safer, more effective alternative to immune diseases.
Given the potential advantages of protein targeted degradation chimera over inhibitors, especially their ability to degrade the entire protein, which may inhibit the entire physiological function of the target protein, in this latest study, the research team explored the BTK-targeting protein targeted degradation chimera degrader L18I in lupus.
First, the research team adoptively transferred BM12 splenocytes into C57BL/6 mice to induce lupus-like autoimmune disease. Treatment with L18I began in the second week of induction and continued for two weeks. Ibrutinib is a BTK inhibitor approved by the US FDA for the treatment of B-cell malignancies. It was used as a positive control due to its therapeutic effect in autoimmune diseases.
The results show that both L18I and ibrutinib can effectively reduce the symptoms of BM12-induced lupus. Specifically, the levels of IgM and IgG autoantibodies, anti-double-stranded DNA, and anti-nuclear antibodies were reduced after treatment. The deposition of antibody immune complexes was also reduced.
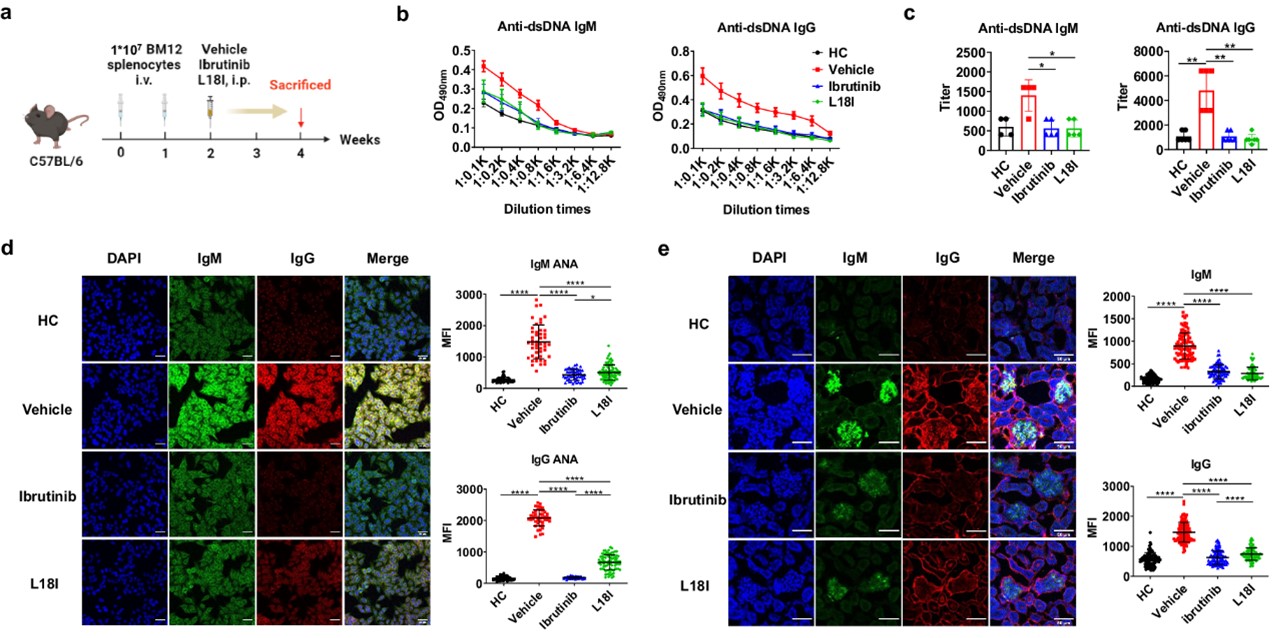
Fig.1 Functional validation of BTK degrader L18I in mouse models.1
Diffuse alveolar hemorrhage (DAH) is an extremely serious complication of lupus disease, with a mortality rate of approximately 50%. It manifests as dyspnea and pulmonary infiltrates, mostly accompanied by kidney disease, elevated anti-double-stranded DNA antibodies, and low complementemia. However, commonly used DAH treatment methods have low specificity and have side effects. Therefore, targeted drugs against DAH are urgently needed. The research team speculates that BTK degraders may have a potential role in the treatment of DAH.
The research team constructed DAH mouse models, which were divided into no DAH, partial DAH, and complete DAH according to lung pathological characteristics, and then treated with different doses of L18I or ibrutinib. L18I significantly reduced the prevalence of DAH compared with the control group, whereas ibrutinib showed only partial effectiveness.
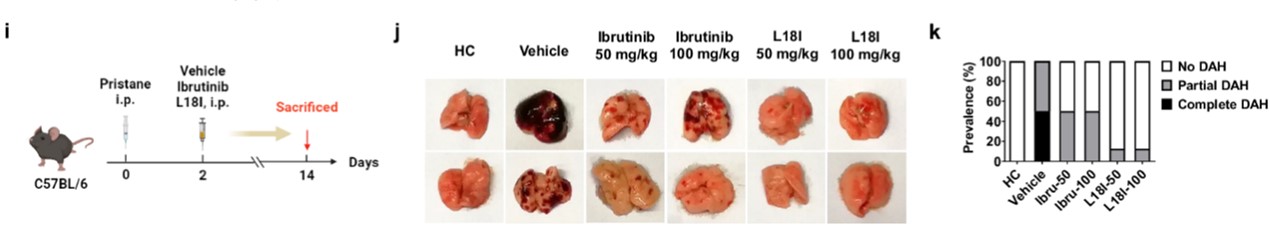
Fig.2 Representative gross images of lung tissue in mouse models.1
For further verification, the DAH score based on HE staining of lung tissue showed that L18I reduced pulmonary hemorrhage and immune cell infiltration, and its effect was more significant than ibrutinib. Consistent with this, lung and spleen weights and total serum IgM levels were reduced after L18I treatment, independent indicators of reduction in DAH syndrome. In addition, L18I also reduced mortality in DAH mice. 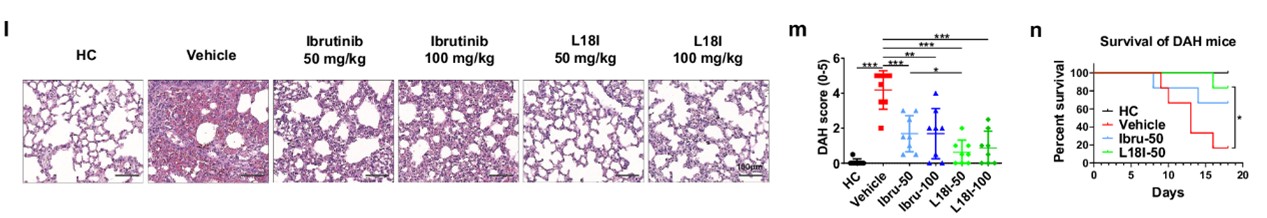
Fig.3 Results of H&E staining of tissues in mouse models.1
The above experimental results show that L18I shows a certain degree of advantage over ibrutinib in relieving symptoms of the DAH mouse model.
During the progression of DAH, myeloid CD11b + Ly6C hi/Ly6C lo mononuclear cell infiltration is thought to be proportional to the severity of DAH. This study showed that both L18I and ibrutinib reduced the proportion and number of myeloid CD11b + Ly6Chi/Ly6C lo monocytes, and the proportion of Ly6C hi monocytes in the lung tissue of mice in the L18I treatment group was slightly lower than that of the ibrutinib treatment group, consistent with the degree of pulmonary hemorrhage. In addition, during the progression of DAH, the proportion of B cells and macrophages in the lungs of mice decreased, but it was reversed to a level almost equivalent to that of healthy mice after L18I treatment, while ibrutinib treatment had no such effect.
The above experimental results show that both L18I and ibrutinib can alleviate the symptoms of DAH in mice, and L18I seems to be more effective than ibrutinib in restoring the normal immune cell microenvironment in the mouse lungs.
In summary, L18I can not only effectively alleviate the symptoms of lupus by reducing antibody secretion, but also alleviate its serious complication diffuse alveolar hemorrhage (DAH) by reducing the inflammatory response. Considering the multiple side effects of Ibrutinib due to off-target effects and the emergence of new mutations leading to treatment non-response, L18I may become a safer and more effective alternative to BTK inhibitors for the treatment of autoimmune diseases.
Reference
- Zhu, Can, et al. “PROTAC for Bruton’s tyrosine kinase degradation alleviates inflammation in autoimmune diseases.” Cell Discovery10.1 (2024): 82.
