Signal transducer and activator of transcription 3 (STAT3) is a member of the STAT family, which responds to a variety of cytokines, growth factors and other signals and activates the expression of downstream genes. STAT3 regulates genes related to survival, proliferation, generation, invasion, metastasis, drug resistance and immune escape in cancer cells. Abnormal regulation of STAT3 can cause a variety of human cancers and other human diseases. Therefore, STAT3 has always been considered as a therapeutic target for diseases such as cancer. Unfortunately, it is difficult to find small molecular drugs targeting STAT3 because of their poor specificity.
A Potent and Selective Small-Molecule Degrader of STAT3 Achieves Complete Tumor Regression In Vivo from a research group of Professor Wang Shaomeng of the University of Michigan, USA, published in Cancer Cell magazine, used Proteolysis targeting chimera technology (PROTAC) technology to design small molecules that can specifically degrade STAT3 in cancer cells both in vitro and in vivo.
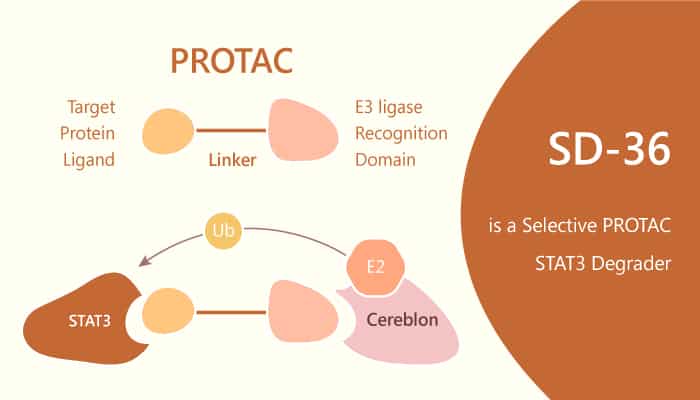
The phosphorylation of STAT3 is critical to its dimerization and subsequent transcriptional activation of target genes. The dimerization of STAT3 depends on the interaction between Src-homology 2 (SH2) domains. Therefore, scientists have been hoping to find small molecular drugs that act on the SH2 domain and thus block STAT3 dimerization and transcriptional activity. However, the clinical function of such small molecular is very limited, case the SH2 domain is very conservative in the STAT family, it is difficult to find STAT3-specific inhibitory small molecules. In order to solve this problem, the authors first carried out structure-oriented optimization design based on the previously found inhibitors of the STAT3 SH2 domain. The small molecular inhibitor SD-36, designed by the authors has high affinity for STAT3 SH2 structure and good cell permeability.
On this basis, SD-36 was modified according to some known factors in the E3 ligase complex, and a ligand of Cereblon (CRBN) / cullin 4A was added to obtain a small molecular PROTAC for STAT3 degradation. Subsequently, in order to test the specific ability of the designed small molecular drug SD-36 to degrade STAT3, the authors used different cell lines and found that the small molecular drug SD-36 could significantly degrade STAT3 in different cell lines and had no significant effect on other members of the STAT family such as STAT1, STAT2, STAT4, STAT5A/B, STAT6 and so on.
Moreover, because STAT3 has been found to have different somatic mutations in a variety of malignant hematological diseases, the mutation rate is as high as 5.3% in blood cancer and lymphatic cancer, especially in the SH2 domain. The authors further tested whether SD-36 could degrade this mutated STAT3. After SD-36 treatment of the cell lines with homozygous mutant STAT3, it was found that SD-36 could degrade not only wild-type STAT3 but also mutant STAT3, effectively. And it was found that the phosphorylation of STAT3 SH2 region had no effect on the degradation of STAT3. Moreover, after the application of SD-36, the authors analyzed the transcriptional activity of STAT in the STAT3-dependent luciferase reporter gene cell line and found that SD-36 specifically affected the transcriptional activity of STAT3 but had no significant effect on the transduction activity of other factors in the STAT family.
Furthermore, the authors found that SD-36 had a significant inhibitory effect on the growth of one leukemia cell line and five lymphoma cell lines after administration of SD-36 in nine leukemia cell lines and nine lymphoma cell lines respectively. At the same time, after analyzing the tumor model of allogeneic transplantation in mice, the authors found that SD-36 could efficiently and selectively degrade STAT3 protein, and had a complete and long-term inhibitory effect on tumor in mice, but had no obvious toxic effect on liver, spleen, heart and kidney. It also had no significant effect on the body weight of mice and the physiological indexes such as albumin, total blood protein, creatinine and blood urea nitrogen in blood.
In general, this study found an effective, highly selective and efficient PROTAC STAT3 inhibitor, which provides an idea for the application of PROTAC technology in other non-pharmacological target design. At the same time, this work has laid a solid foundation for STAT3 as a target for clinical drug development, and the discovery of STAT3 degradation drugs provides a new idea for the treatment of human cancer and other human diseases.
