As a type of antibody with good application prospects, single domain antibodies have advantages in the diagnosis and treatment of many diseases. However, its short half-life in plasma limits its application in the therapeutic field. The half-life refers to the time required for the drug concentration in plasma to drop to half after the injection is completed. This article will discuss how to regulate the half-life of single domain antibodies to improve their application value in the field of therapy.
Why do single domain antibodies have a shorter half-life than traditional antibodies?
There are two main reasons for the short half-life of single domain antibodies:
- Glomerular filtration results in rapid clearance of single domain antibodies from the body, and the limit of glomerular filtration protein drugs is generally around 60 kDa. That is to say, small molecular weight antibody fragments or derivatives, such as single domain antibody (15 kDa) and scFv (28 kDa) or even Fab (50 kDa), will be cleared from the body through glomerular filtration, resulting in a short half-life. Among them, single domain antibodies have the smallest molecular weight among all antibody types, and their half-life is often only tens of minutes.
- Single domain antibodies lack the ability to specifically bind to FcRn. Traditional antibodies usually have a longer half-life because of their larger molecular weight and the ability to specifically bind to FcRn (neonatal Fc receptor). FcRn can protect the antibody from being degraded by lysosomes, and then the antibody is transported to the surface of the cell membrane with FcRn, loses its combination with FcRn, and is re-released into the plasma, thereby prolonging the half-life of the antibody.
Strategies to regulate the half-life of single domain antibodies
- Increase the molecular weight: Increase the molecular weight of single domain antibodies through fusion proteins, such as HSA (human serum albumin) or multimer structures, and reduce the renal clearance rate.
- Combining with Fc fragments: Combining single domain antibodies with Fc fragments to extend its half-life by borrowing the function of FcRn.
- PEGylation: Modification of single domain antibodies with polyethylene glycol to prolong their circulation in the body.
- Liposome encapsulation: Encapsulate the single domain antibody in liposomes through nanotechnology to improve its stability and prolong its half-life.
At present, single domain antibody drugs that have entered the clinic or have been marketed mainly use the method of fusing Fc, HSA, or HSA single domain antibodies to prolong the half-life.
Example
Example 1—Half-life extension by fusion of anti-HSA single domain antibodies
HSA is the most abundant and most stable protein in human plasma, containing 585 amino acids and a molecular weight of about 66.5 kDa. The X-ray crystal structure of HSA shows that it is a heart-shaped molecule consisting mainly of α-helices and lacking β-sheets. It contains three homologous domains, DI, DII, and DIII, each of which is subdivided into A and B subdomains (DIA, DIB, DIIA, DIIB, DIIIA, and DIIIB). These domains are connected by long and flexible loops. Plasma concentrations of HSA are approximately 45 mg/ml (0.6 mM), with a circulating half-life of up to 20 days. When single domain antibodies are combined with HSA, their hydrodynamic radius and molecular weight will increase, glomerular filtration will be reduced, and their residence time in vivo will be greatly extended.
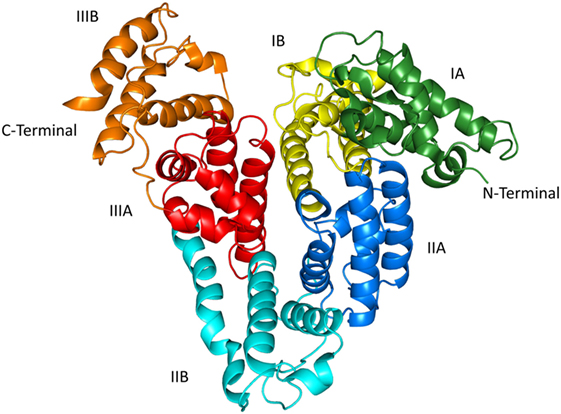
Figure 1. X-ray crystal structure of HSA (PDB ID: 1uor)
Example 2—Extending the half-life of single domain antibodies by binding to Fc fragments
Antibody Fc fragments are constant regions of antibodies in which the amino acid sequence remains unchanged in different types of antibodies. The antibody type is determined by the specific structure of the FC fragment. Unlike monoclonal antibodies, single domain antibodies cannot achieve antibody-dependent cell-mediated cytotoxicity (ADCC) effects and serum stability due to the lack of Fc fragments, which require the participation of Fc receptors. The Fc receptor FcγRIIIa (CD16a) plays a key role in natural killer cells (NK)-mediated ADCC. By conjugating a single domain antibody to a recombinant humanized heavy-chain antibody form of the Fc fragment, its Fc-mediated effector function and blood residence time can be increased, but its diffusion advantage can also be reduced. IgG1 Fc was chosen as the fusion fragment of the single domain antibody because of the affinity of IgG1 and the Fc receptor protein.
Example 3—Multivalent Single Domain Antibodies
Multivalent single domain antibodies have a larger molecular weight and are effective against glomerular filtration.
Example 4—Extending the half-life of single domain antibodies by PEGylation
Polyethylene glycol (PEG for short) is formed by the polymerization of ethylene glycol and belongs to the class of polyether substances. It has good water solubility, extremely low immunogenicity and toxicity. PEGylation refers to the covalent combination of drugs with PEG to improve the pharmacokinetics, pharmacodynamics, and immunological properties of drugs and improve their therapeutic effects. PEGylation can change the physical and chemical properties of drugs, enhance the retention time of drugs in vivo, prolong the half-life, and improve the binding affinity of cell receptors, thereby improving tumor targeting and reducing the incidence of adverse events. PEG can also improve the solubility and stability of proteins, which is helpful for the production and storage of drugs. Therefore, PEG is widely used in drug delivery and drug modification technologies and can be directly coupled with drugs or attached to the surface of drugs and encapsulated in nanomaterials.
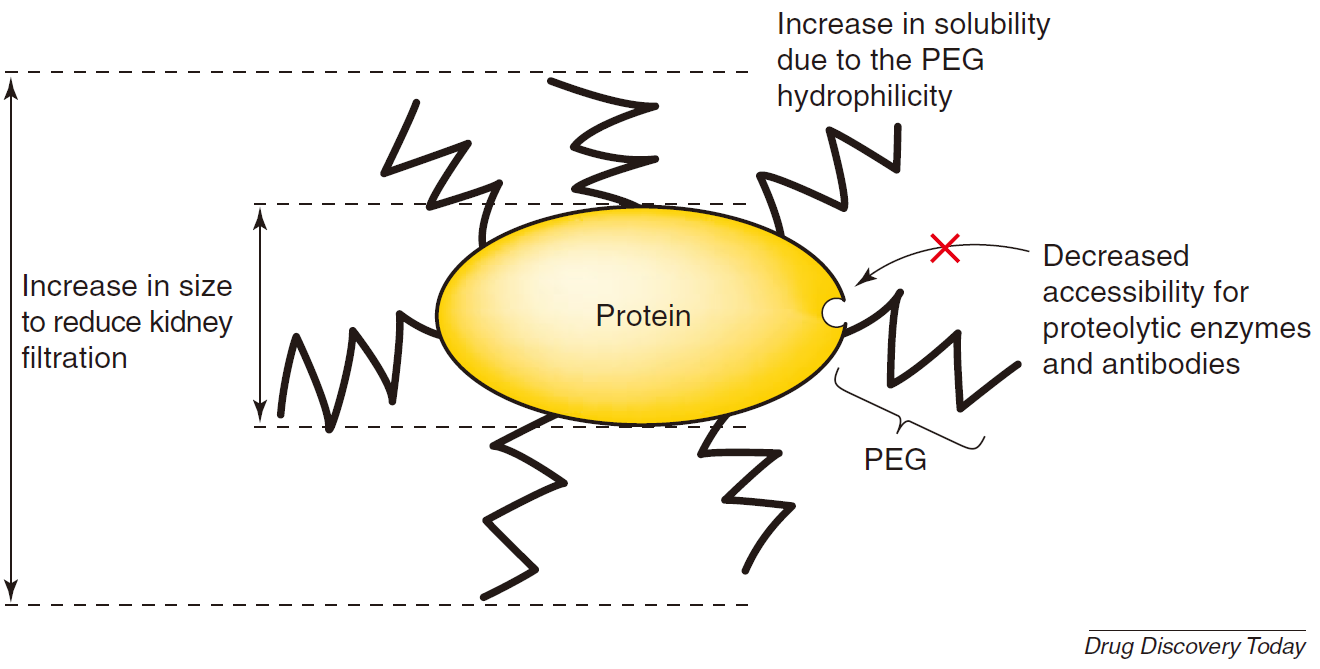
Figure 2. Main advantages of PEGylated protein (Francesco M. Veronese, 2005)
Due to the small molecular weight and short half-life of single domain antibodies, their performance in clinical applications is limited. Therefore, regulating the half-life of single domain antibodies is of great significance for expanding their application in the therapeutic field. At present, there are many methods that can effectively increase the half-life of single domain antibodies, such as increasing the molecular weight, combining with Fc fragments, fusing carrier proteins or anti-HSA single domain antibodies, pegylation, and liposome encapsulation. Some methods have entered the clinical trial stage or have been approved for marketing. We expect more single domain antibody drugs to be promoted to the clinic and marketed to promote the development of human health.
Reference
Chaudhury C, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003 Feb 3;197(3):315-22. doi: 10.1084/jem.20021829.
Coppieters K, et al. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 2006 Jun;54(6):1856-66. doi: 10.1002/art.21827.
Hua, B. et al. A novel single domain antibody targeting TIGIT for cancer use in combination therapies. Cancer Research 81, 2451-2451 (2021).
