Single domain antibodies are derived from llama heavy chain-only antibodies (HCAbs). They represent a new generation of biologics with unique properties. Single domain antibodies have excellent tissue distribution, high temperature and pH stability, are easy to produce recombinantly, and can be easily converted into different formats. In addition, single domain antibodies have the unique ability to bind molecular clefts, such as the active site of an enzyme, thereby interfering with the function of the target protein. Over the past decade, a number of single domain antibodies against inflammation-related proteins have been developed with the aim of modulating their immune function. Here, we outline recently developed single domain antibodies targeting immune pathways associated with neuroinflammation. Furthermore, we highlight strategies to modify single domain antibodies, which enable them to overcome the blood-brain barrier and serve as highly specific therapies for acute inflammatory brain injury.
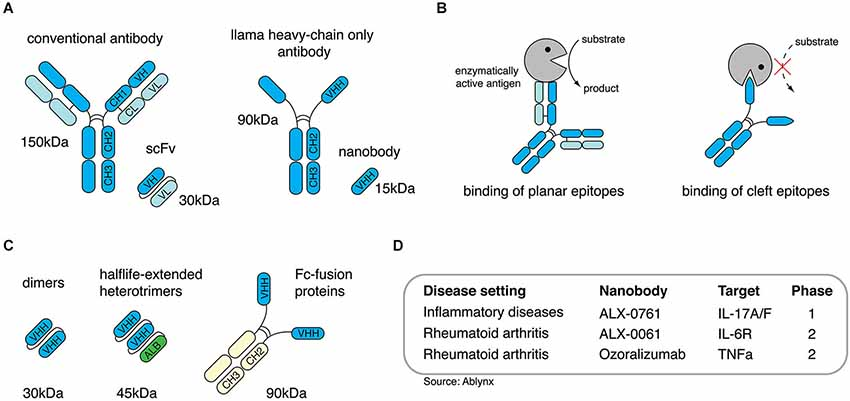
Single domain antibodies are derived from llama heavy chain-only antibodies (HCAbs)
To combat infectious diseases, many single domain antibodies against bacterial and viral antigens have been produced to prevent or improve pathogenicity. More recently, key players in immune pathways have become targets of single domain antibodies to modulate immune responses. This has generated single domain antibodies against Fc receptors (FcRs), chemokine receptors, chemokines, cytokines and extracellular enzymes. These single domain antibodies generally exhibit high target specificity and can modulate the function of their targets in an agonistic or antagonistic manner.
In 2010, Martine Smit’s group reported the generation of two single domain antibodies that specifically target the chemokine receptor CXCR4. Single domain antibodies 238D2 and 238D4 showed potent competitive inhibition of CXCL12 binding to CXCR4. When injected into monkeys, anti-CXCR4 single domain antibodies induced the mobilization of hematopoietic stem cells by disrupting the CXCR4/CXCL12 axis that contributes to hematopoietic stem cell residency in the bone marrow. In 2013, the same group reported the generation of antagonistic single domain antibodies against CXCR7. When injected into mice, these single domain antibodies showed beneficial effects in an in vivo xenograft model of head and neck cancer. Meanwhile, the same group published a set of single domain antibodies specifically targeting CCL2, CCL5, CXCL11 and CXCL12. The binding of single domain antibodies to CXCL11 and CXCL12 inhibited chemokine receptor binding, thereby preventing in vitro cell migration induced by chemokine receptor activation. Since a variety of chemokines and their receptors are known to facilitate the migration of immune cells to the brain following brain injury, the single domain antibodies described above may be promising therapeutic alternatives for the treatment of acute brain injury. Muruganadam et al. in 2002 described the selection of a single domain antibody (FC5) that crossed human blood-brain barrier endothelial cells in vitro. Later, the same group proposed that FC5 binds to the putative α(2,3)-sialoglycoprotein receptor and is transcytosed via clathrin vesicles. The results demonstrate that FC5 conjugated to the opioid peptide Dal can be used as an in vivo drug delivery shuttle to induce significant analgesic responses compared to the unconjugated Dal peptide. Other approaches utilize receptor-mediated transcytosis for brain targeting. In addition, transferrin receptors and insulin receptors are also used for receptor-mediated endocytosis of small-molecule drugs and therapeutic proteins. These studies suggest that single domain antibodies that bind to these receptors and trigger transcytosis may be a promising alternative for ligand-based drug delivery to the brain. A study by Pierre Lafaye’s group reports that single domain antibodies with a high electrical point (pI) can spontaneously cross the blood-brain barrier. Such single domain antibodies can not only enter the brain, but can even penetrate cells and bind to intracellular proteins.
After acute brain injury, such as ischemic stroke or trauma, danger-associated molecular patterns (DAMPs) released by necrotic cells activate resident microglia, leading to the production of pro-inflammatory mediators such as cytokines and chemokines that attract other immune cells. Preventing local inflammation may be a means of preventing further loss of brain tissue. High mobility group box 1 (HMGB1) and nucleotide DAMPs such as adenosine triphosphate (ATP) are released during cerebral ischemia. Antibody-mediated neutralization of HMGB1 or its receptor antagonism significantly reduced infarct size in middle cerebral artery occlusion in a mouse model of ischemia/reperfusion. The ATP receptor P2X7, which mediates inflammasome formation and cell death, was antagonized by small-molecule inhibitors in a mouse model of transient focal ischemia, again resulting in reduced infarct size. Both the HMGB1/RAGE and ATP/P2X7 pathways show promising targets for single domain antibody-mediated antagonism.
Single domain antibodies have been shown to be versatile and effective biologics suitable for the treatment of inflammatory diseases. Due to their unique structure, single domain antibodies have the potential to modulate the function of cell surface and secreted proteins in an agonistic or antagonistic manner. They can be genetically engineered to extend their half-life in the body, and in proof-of-concept studies, they have been shown to act as shuttles for the delivery of therapeutic agents through the BBB. Future studies will have to demonstrate whether this strategy can also be used to deliver therapeutic single domain antibodies to the brain to improve neuroinflammation outcomes.
