The team of Professor Michael J. Mitchell of the University of Pennsylvania and Professor Drew Weissman, winner of the 2023 Nobel Prize in Physiology and Medicine, published a review paper titled “Enhancing in situ cancer vaccines using delivery technologies” in the journal Nature Reviews Drug Discovery.
The review starts from the cancer immune cycle and systematically summarizes solid tumor drugs and gene delivery technologies that enhance the effect of in situ vaccines. Starting from three dimensions: promoting the release of tumor antigens, enhancing tumor antigen processing and presentation, and overcoming the inhibitory tumor microenvironment, delivery technologies of different categories, different characteristics, and targeting different regions and cell types are summarized and analyzed. In addition, this review also systematically summarizes the research progress of tumor in situ vaccines based on delivery technology currently in the clinical trial stage. Finally, while affirming the development prospects of tumor in situ vaccines, the researchers outlined their existing clinical problems and the prospects for developing new vaccine delivery systems.
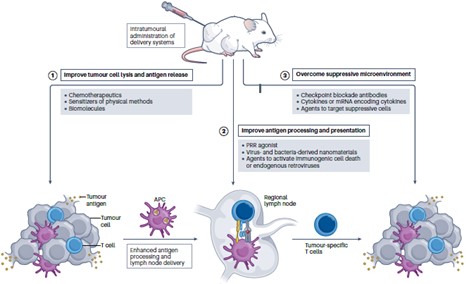
Fig. 1 Improving in situ tumor vaccines using delivery technology.1
- Promote tumor antigen release
Inducing tumor cell lysis and death can release tumor antigens, but methods such as the sole use of chemotherapy drugs, physical induction, and biomolecule induction all have limitations. This article analyzes how modified delivery, delivery, and co-delivery technologies can address these issues and promote tumor antigen release through in situ tumor vaccines.
- Chemotherapy drug delivery technologies include PEG modification to improve tissue distribution, active targeting ligand connection to achieve targeted delivery, pH-responsive specific delivery, and local chemotherapeutic agent delivery. Such nanoparticle-mediated chemotherapeutic drug delivery is based on tumor targeting. Site-specific delivery can effectively improve drug accumulation in tumors.
- Delivering nanosensitizers mainly includes designing delivery materials as sensitizers for radiation, photodynamic therapy (PDT), sonodynamic therapy (SDT), and physical therapy methods such as microwave, ultrasound, and cryoablation therapy, to solve the problem of tumors. Low cell lysis efficiency and extratumoral toxicity are associated with induction by physical methods.
- The delivery of biomolecules can destroy cell membranes or regulate cell death to induce tumor cell lysis, broadening the application scope of biomacromolecules, including oncolytic viruses and cytolytic peptides.
- Enhanced tumor antigen processing and presentation
Antigen-presenting cell (APC)-mediated processing and presentation of tumor antigens can be improved through activation of pattern recognition receptors (PRRs). Next, we discuss how delivery technologies can be used to improve antigen processing and presentation by dendritic cells through direct and indirect activation of PRRs.
On the one hand, agonists that deliver PRRs can be used to directly activate the innate immune response, including the delivery of synthetic PRR agonists, the delivery of nanoparticles of virus-derived materials or bacterial-derived materials, etc. These reagents can be recognized by PRRs, such as Toll-like receptors (TLRs), NOD-like receptors (NLRs), or cyclic GMP-AMP synthetase-interferon gene stimulator (cGAS-STING), stimulating antigen processing and presentation.
In addition, PRRs can be activated indirectly using delivery methods such as targeting immunogenic cell death (ICD) or targeting endogenous retroviral (ERV) genes. Delivery of targeted ICD activation reagents does not directly activate PRRs, but causes tumor cells to release tumor antigens and damage-associated molecular patterns (DAMPs), which can be recognized by PRRs and activate innate immune responses. Similarly, nanoparticles used to deliver epigenetic drugs (DNMTis or HDACis, etc.) can activate ERV genes in cancer cells, leading to the formation of ssRNA, dsRNA, and dsDNA, which can also trigger innate immune responses after being recognized by PRRs.
Related Products
| CAT | Product Name | Type |
| VAdv-Ly0016 | TLR2 & NOD2-Based Adjuvant | PRR Agonist |
| VAdv-Ly0018 | Imidazoquinoline (TLR7 Agonist) Antigen Grade | PRR Agonist |
| VAdv-Ly0019 | Cyclic [G(2′, 5′)pA(3′, 5′)p] | PRR Agonist |
| VAdv-Ly0020 | 3’3′-cGAMP | PRR Agonist |
| VAdv-Ly0021 | Cyclic Diadenylate Monophosphate | PRR Agonist |
Delivery technologies to improve tumor antigen processing and presentation
- Overcoming the immunosuppressive tumor microenvironment
Solid tumors have a highly immunosuppressive tumor microenvironment (TME). This review discusses how delivery technologies targeting immune checkpoints, cytokines, and suppressive immune cells in the suppressive TME can be used to improve in situ tumor vaccines.
- Target immune checkpoints. For example, local delivery of immune checkpoint inhibitors that synergize with in situ vaccines and target the CD47-SIRPa axis can improve vaccine efficacy and reduce adverse effects caused by systemic immune checkpoint blockade through local delivery and stimulus-responsive delivery technology. Reactions are minimized.
- Immunomodulation by local delivery of cytokines. This method includes direct delivery of cytokines (IL-2, IL-12, IL-15, etc.) or delivery of nanoparticles encoding various cytokine mRNAs. Compared with traditional cytokine administration routes (such as intravenous injection ), this method can improve efficacy and reduce systemic toxicity, improving the effect of in situ tumor vaccines.
- Regulate suppressive immune cells. The tumor microenvironment can cause the accumulation of immunosuppressive cells such as Treg cells, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs). Designing delivery systems that deliver small molecules to deplete suppressive immune cells or deliver therapeutic RNA to remodel immune suppressive cells can enhance the efficacy of in situ tumor vaccines.
- Target metabolism. For example, injecting compounds that regulate metabolism into tumors is intended to enhance anti -tumor immunity by manipulating the metabolism of cancer cells and immune cells that infiltrate tumors.
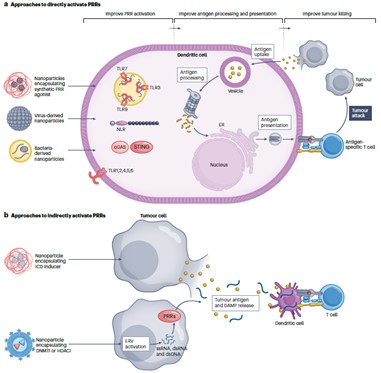
Fig. 2 Delivery technologies to overcome the suppressive immune microenvironment. 1
- Clinical research
This review also provides a systematic summary of in situ tumor vaccine delivery systems currently undergoing clinical evaluation. In order to improve the release of tumor antigens, clinical trials of INT230-6 and the radiosensitizer Hensify, alone or in combination with immune checkpoint blockade therapy, for the treatment of various types of cancer are ongoing. While drugs in clinical trials, such as Hiltonol, BO-112, G100, and CMP-001, can improve antigen processing and presentation. To overcome the suppressive immune microenvironment, clinical trials are currently underway on mRNA delivery systems encoding cytokines, including MEDI1191, mRNA-2416, mRNA-2752, and SAR44100. In summary, more and more clinical studies are ongoing to explore in situ tumor vaccines based on delivery technology.
Summary and Outlook
Tumor in situ vaccines have significant advantages because they can avoid the need for antigen identification and isolation and solve the problem of tumor heterogeneity. Many drugs are under clinical evaluation, and some products have been approved. They have great prospects in the treatment of solid tumors. Currently, more and more delivery technologies can be used to enhance the clinical therapeutic effect of in situ vaccines.
In the future, it is still expected to further develop the delivery technology of in situ vaccines for tumors through the following approaches: first, there is an urgent need for delivery technology that can specifically capture tumor antigens and deliver them to APC to induce specific immune responses; second, it should be based on immune The safety characteristics of modulators are focused on developing a more durable sustained-release strategy for in situ tumor vaccines; the third is to gradually optimize the efficacy of local immunotherapy or combination therapy and overcome the accessibility challenges of in situ vaccines to tumor lesions.
Taken together, with continuous improvement, these delivery systems will significantly enhance the effectiveness of existing in situ vaccine-based cancer treatments and create more advanced cancer immunotherapies, which are expected to have great potential in basic immunology research and clinical use of in situ vaccination in the future. are more widely used in the environment.
Reference
- Gong, Ningqiang, et al. “Enhancing in situ cancer vaccines using delivery technologies.” Nature Reviews Drug Discovery(2024): 1-19.
