Janssen Pharmaceutical recently announced that the US Food and Drug Administration (FDA) has awarded the company the breakthrough drug qualification (BTD) for prophylactic RSV advanced vaccines. It is used to prevent lower respiratory tract diseases mediated by respiratory syncytial virus (RSV) in people aged 60 and over. The elderly population is one of the populations with the highest risk of RSV infection.
BTD, a new drug review channel created by FDA in 2012, aims to accelerate the development and review of new drugs which used to treat serious or life-threatening diseases and has preliminary clinical evidence that can substantially improve the condition compared with existing therapeutic drugs. BTD-qualified drugs can be developed with closer guidance from senior FDA officials, to ensure that new treatment options are available to patients in the shortest possible time.
The BTD award is based on clinical data of the Janssen prophylactic RSV advanced vaccine, which may show a significant improvement in clinical effective endpoints compared with the currently available nursing standards. At present, the vaccine is in a Phase IIb proof-of-concept study to investigate the safety and effectiveness of preventing RSV infection in people aged 65 and older.
Johan van Hoof, Managing Director of Janssen Vaccine and Prevention, head of Global treatment at Janssen Pharmacy, said: “due to the lack of preventive vaccines or effective antiviral treatments, RSV remains an important cause of illness among high-risk groups, especially the elderly. This groundbreaking drug qualification represents a clear recognition of the transformative potential of our RSV preventive solutions. We look forward to working closely with the FDA throughout the preventive RSV advanced vaccine development project. “
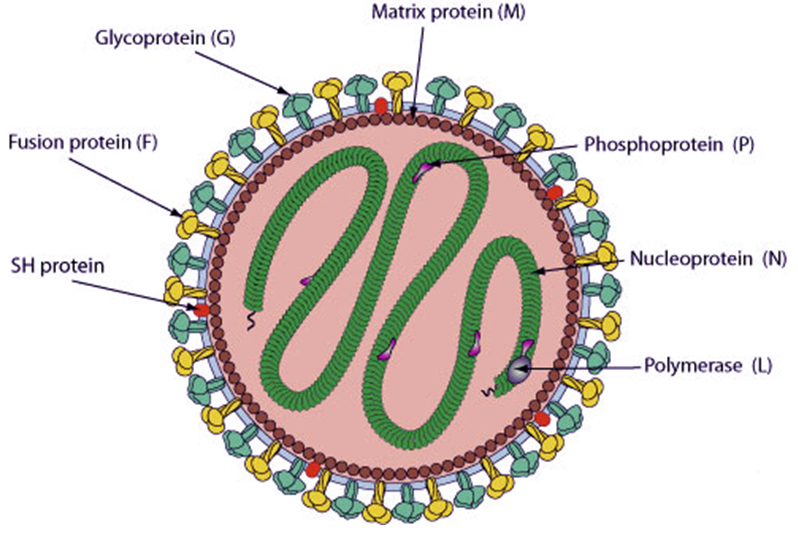
Respiratory Syncytial Virus (RSV)
Respiratory syncytial virus (RSV) is a highly epidemic and highly contagious respiratory tract infection, which is the main cause of bronchitis and pneumonia, affecting more than 64 million people worldwide every year. Because the symptoms of RSV infection are difficult to distinguish from influenza or other respiratory diseases, many people infected with RSV may not be able to diagnose correctly. Among the elderly, in the United States alone, RSV hospitalized 177000 people and killed 14000 each year. In the absence of prophylactic vaccines or effective antiviral drugs, RSV is a huge health and economic burden on a global scale. In the United States, the cost of hospitalization caused by RSV for people 65 and older is estimated at more than $1 billion a year.
The prophylactic RSV advanced vaccine, which uses the fusion protein-encoding RSV virus as the antigen, is developed through the adenoviral vector platform and is being evaluated for the prevention of RSV-mediated lower respiratory diseases in people aged 60 and older.
