Vaccines have transformed modern medicine, yet their impact hinges on a behind-the-scenes hero: analytical development. This process ensures every dose meets strict safety and efficacy benchmarks, from early design to global distribution. Let’s explore the cutting-edge tools and challenges shaping this field—and how scientists are pushing boundaries to deliver better vaccines faster.
The Science Behind Vaccine Quality Control
At its core, analytical development answers one question: Does this vaccine work consistently and safely? To find out, researchers deploy over 100 tests assessing everything from antigen purity to stability under stress. Take mRNA vaccines, for example. Their fragile nature demands real-time monitoring during production. Techniques like mass spectrometry now track critical modifications in lipid nanoparticles, ensuring they protect the genetic payload until delivery.
Validation is equally rigorous. Before any test becomes routine, it must prove its accuracy across labs and conditions. Regulatory agencies demand this step—after all, a slight variation in pH or temperature could skew results. Recent strides in automation help here, minimizing human error in repetitive tasks like potency assays.
From Lab Bench to Clinic: Key Development Milestones
- Preclinical Testing: This phase starts with in vitro assessment—think of it as a “test tube preview” of immune responses. Neutralization assays, for instance, mimic how antibodies block viruses in cell cultures. But cells alone can’t predict real-world outcomes. That’s where in vivo assessment in animals bridges the gap. Studies in ferrets helped validate COVID-19 vaccines’ ability to reduce viral shedding, a crucial clue for human efficacy.
- Clinical Trials: Phase I trials focus on safety in small groups, while Phase III trials—like those for recent malaria vaccines—enroll thousands to confirm protection across ages and geographies.
- Post-Market Vigilance: Even after approval, monitoring continues. Denmark’s nationwide health registries, for example, detected rare myocarditis cases linked to mRNA vaccines, leading to updated risk guidelines.
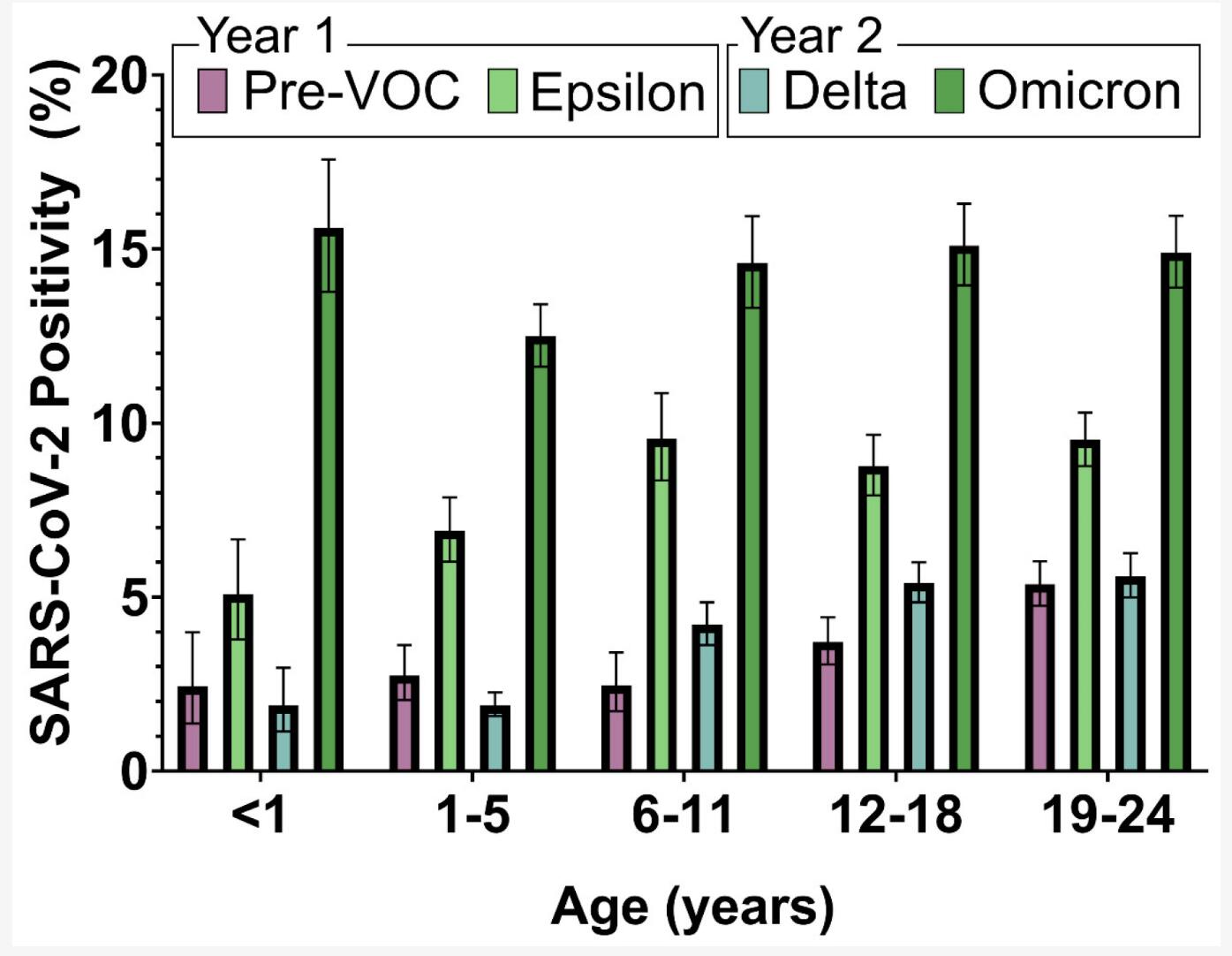
Figure 1. Percent of positive SARS-CoV-2 tests by age.1
Tech Trends Reshaping Vaccine Analysis
The field is embracing tools that would’ve seemed sci-fi a decade ago:
- AI’s Growing Role: Machine learning now predicts which viral regions trigger strong immune responses. In 2023, researchers used AI to map conserved epitopes in flu variants, slashing design time for universal vaccines.
- Imaging at Atomic Scale: Cryo-electron microscopy lets scientists “zoom in” on vaccine components, like the spike proteins of coronaviruses, ensuring each batch matches the intended structure.
- Omics Integration: By combining proteomics (protein analysis) with immune profiling, teams can pinpoint why some people respond poorly to vaccines—and tweak formulations accordingly.
These advances are vital for complex candidates, like cancer vaccines targeting neoantigens unique to individual tumors.
Hurdles on the Road to Equity
Progress hasn’t erased old challenges:
- Evolving Pathogens: When SARS-CoV-2’s Omicron variant emerged, labs raced to update neutralization tests. Many now use pseudo-viruses engineered with variant spikes, avoiding risky live-virus work.
- Scaling Up Gracefully: Viral vector production, crucial for Ebola and HIV vaccines, often faces low yields. New bioreactor designs that mimic human cell environments are boosting output.
- Global Standardization: A 2022 study found 30% variability in adjuvant testing methods between labs. Groups like the WHO are pushing for unified protocols, especially for neglected diseases.
What’s Next? A Glimpse into Tomorrow’s Vaccines
- No More Freezers? Lyophilized mRNA vaccines, stable at room temperature, could revolutionize distribution in tropical regions.
- Tailored Immunity: Gene-edited antigens might one day personalize vaccines for immunocompromised patients.
- Eco-Friendly Production: Algae-based expression systems are being explored to grow vaccine proteins sustainably.
Wrapping Up
Analytical development isn’t just about checkboxes—it’s where creativity meets rigor. Whether through AI-guided designs or meticulous in vitro and in vivo assessments, each innovation brings us closer to vaccines that are safer, more effective, and accessible to all. As pathogens evolve and health disparities persist, this field remains our best defense in the endless arms race against disease.
Comprehensive Vaccine Analytical & Safety Testing Solutions at Creative Biolabs
At Creative Biolabs, we provide end-to-end analytical and preclinical testing services to ensure the safety, efficacy, and compliance of your vaccine candidates. Our GLP/GMP-compliant platforms support every stage of development, from early-stage characterization to regulatory submissions.
- Vaccine Analytical Development & Qualification
- Vaccine Analytical Development & Qualification: Comprehensive physicochemical and biological characterization.
- Vaccine Identity Tests: Confirm antigen specificity and structural integrity.
- Vaccine Purity Tests: Assess contaminants, excipients, and process-related impurities.
- Vaccine Stability Tests: Real-time and accelerated stability studies under ICH guidelines.
- In Vitro & In Vivo Efficacy Assessment
- In Vitro Vaccine Assays: Cell-based potency, neutralizing antibody, and immunogenicity testing.
- Influenza Vaccine Potency Testing: Hemagglutination (HA) and single radial immunodiffusion (SRID) assays.
- In Vivo Vaccine Evaluation: Animal models for immunogenicity and protective efficacy.
- Safety & Toxicology Studies
- GLP Toxicology & Safety Testing: Repeat-dose, local tolerance, and hypersensitivity studies.
- GLP In Vivo ADME/PK Studies: Pharmacokinetic profiling of vaccine components.
- GLP Reproductive Toxicology: Developmental and fertility safety assessments.
- Microbial Quality Control
- Vaccine Microbial Tests: Sterility, endotoxin, and bioburden testing per USP/EP standards.
Why Partner With Us?
- Regulatory Expertise: Protocols aligned with FDA, EMA, and WHO requirements.
- Customized Solutions: Tailored assays for novel vaccine platforms (mRNA, viral vectors, etc.).
- Fast Turnaround: Accelerated timelines for critical milestones.
- Full Traceability: Audit-ready documentation and data integrity.
From preclinical development to IND-enabling studies, Creative Biolabs is your trusted partner to de-risk vaccine development and ensure regulatory success.
Contact us today to discuss your project needs and access our end-to-end testing capabilities!
Reference
Saleh, T.; Fuller, T.; Cambou, M.C.; et.al. Epidemiology and SARS-CoV-2 Infection Patterns among Youth Followed at a Large Los Angeles Health Network during 2020–2022: Clinical Presentation, Prevalent Strains, and Correlates of Disease Severity. Vaccines 2023, 11, 1024. https://doi.org/10.3390/vaccines11061024. Distributed under Open Access license CC BY 4.0, without modification.
