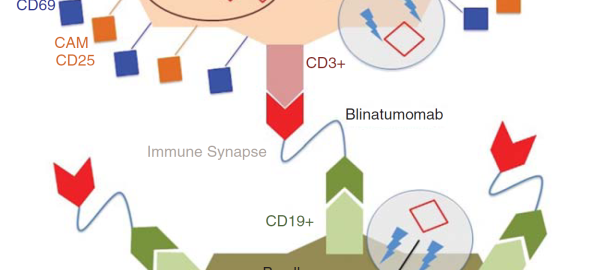
Blinatumomab is a CD19/CD3 bispecific antibody that is the first bispecific T cell engager (BiTE) and the first CD19-specific antibody approved by the U.S. Food and Drug Administration (FDA). As an immunotherapyRead More…
Targeted Killing of Breast Cancer Cells by p95HER2-T Cell Bispecific Antibody
Immunotherapy was positioned as an emerging and even promising treatment for cancer. Today’s new immunotherapeutic agents for different tumor types, whether used alone or in combination, are increasingly becoming one of theRead More…
The World’s First-and-Only Bispecific T Cell Engager Immunotherapy Approved in Japan
Amgen, the United States biotechnology giant, recently announced that the Ministry of Health, Labor and Welfare (MHLW) has approved bispecific T cell engager immunotherapy Blincyto® (blinatumomab) for the treatment of relapsed orRead More…