CAR-T (chimeric-antigen receptor T-cell immunotherapy) has significant efficacy in the treatment of acute leukemia and non-Hodgkin’s lymphoma, and is considered to be one of the most promising tumor treatments. In recent years, CAR-T therapy has been gradually improved. In addition to the aforementioned blood cancer, CAR-T has also been used to treat diseases such as solid tumors, autoimmune diseases, HIV infection, and heart disease. This article summarizes the latest significant advances in CAR-T cell therapy as of May 2022.
CAR-T Combined with Azacitidine—More Effective in the Treatment of AML
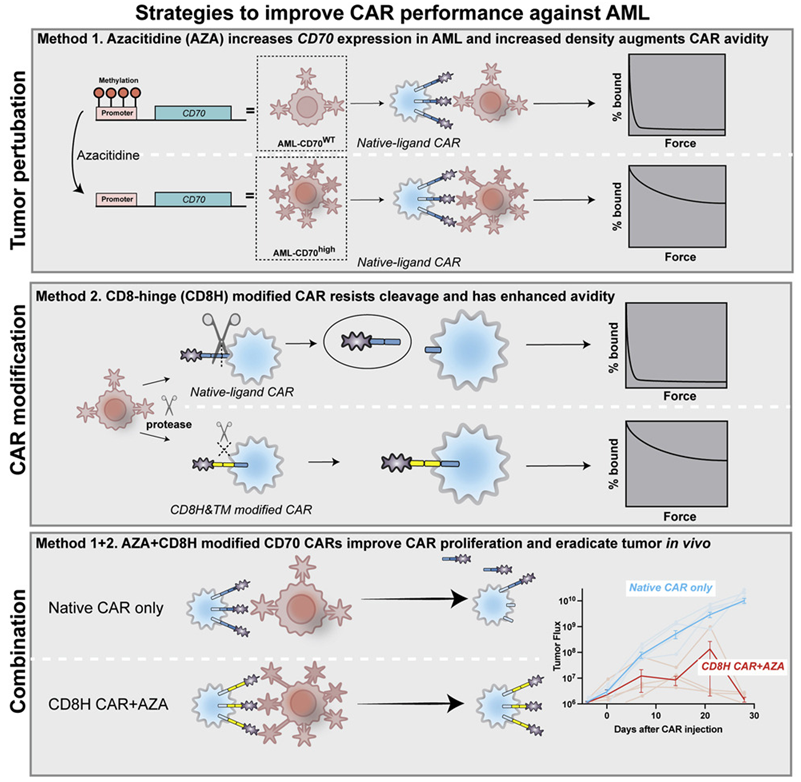
Acute myeloid leukemia (AML) is the most common form of leukemia in adults. In a new study, researchers from Massachusetts General Hospital (MGH) have developed a new strategy to treat AML, which involves utilizing a drug to expand the number of target antigens on the surface of cancer cells, and a genetic modification approach to allow T cells to attach more tightly and persistently to these target antigens. It has the potential to come into the market and bring benefits to AML patients. The related research results were published online in Cancer Cell on April 21, 2022, with the title of “Non-cleavable hinge enhances avidity and expansion of CAR-T cells for acute myeloid leukemia”.
Previous attempts to treat advanced AML with CAR-T cell therapy have been hampered by the lack of suitable antigens. The first author of the paper, Dr. Mark B. Leick, researcher of the Cellular Immunotherapy Program at MGH Cancer Center, together with the corresponding author, Dr. Marcela V. Maus, Director of Cellular Immunotherapy at MGH Cancer Center, and his colleagues, used a CD70 antigen-targeting CAR-T cells (CD70 CAR-T cells) in this study. CD70 antigen is more abundant on AML cells than on normal bone marrow cells. Using only CD70 CAR-T cells was modestly effective against AML in animal models, and combining it with the FDA-approved AML drug azacitidine increased the generation of CD70 antigens on the surface of cancer cells. Their study shows that combining the drug with CD70 CAR-T cells achieved better effect of killing tumor cells.
Furthermore, unlike most CARs targeting an antigen using mouse antibodies with chances of eliciting unwanted immune responses, the CAR used in this study relies on a natural ligand molecule that binds tightly to the antigen, thereby avoiding the possibility that the immune system would recognize the tumor-killing CAR-T cells as invaders and try to reject them.
Smarter CAR-T Cell Therapy—CAR-T Cells Expressing Modular SNIPR Receptor
Therapies based on engineered immune cells have recently emerged as a promising approach to treating cancer. Engineered immune cells are more precise and sophisticated in their ability to detect and eliminate cancer cells than traditional drugs. However, despite their promise, cell-based therapies still face limitations, including toxicity and the possibility that they may attack healthy cells. In addition, scientists do not have a good grasp of how to genetically modify existing therapeutic cells to expand their applications or better control their activity.
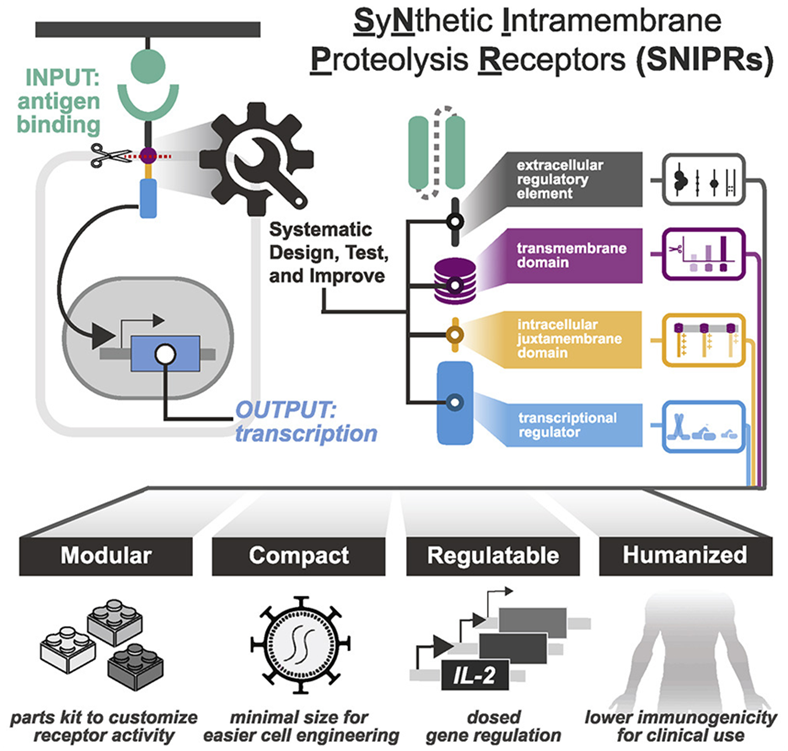
To overcome these limitations, researchers at the Gladstone Institute and UCSF in a new study systematically analyze the molecular building blocks used to engineer therapeutic cells. Their research creates a comprehensive rulebook for designing therapeutic cells with greater specificity and safety, and ultimately customizing cell-based therapies. The results of the study were published in the April 14, 2022 issue of the journal Cell, with the title “Modular design of synthetic receptors for programmed gene regulation in cell therapies”.
The corresponding author Kole Roybal, Ph.D., associate professor in the Department of Microbiology and Immunology at UCSF, and his team constructed a catalog of receptors they call SNIPR (synthetic intramembrane proteolysis receptor), which are small enough to be inexpensively engineered in human cells. They are also made entirely of human receptor fragments and can detect and respond to small numbers of targets. In addition, the activity of SNIPRs can be tuned so that the cells that carry them not only kill target cells, but also deliver specific molecules to precise disease locations.
The authors evaluated the ability of these optimized receptors to clear tumors in mouse models of leukemia, mesothelioma, and ovarian cancer. To reduce the chance of killing off-target cells, they combined SNIPR, which is designed to recognize one molecule on the surface of a tumor, with a CAR receptor that has been genetically modified to recognize another tumor molecule. Furthermore, they made CAR receptor production dependent on SNIPR receptor activation. In this way, only cells that carry the targets of synNotch and the CAR receptors were killed, while cells that carry only one of these targets were not.
Combining Oncolytic Cells with CAR-T Cell Therapy Improves Treatment of Solid Tumors
In a new study, researchers from the Mayo Clinic designed an immunotherapy technique that combined CAR-T cell therapy with an oncolytic virus to more effectively target and treat solid tumors. The findings were published in the April 13, 2022 issue of Science Translational Medicine, with the title “Oncolytic virus–mediated expansion of dual-specific CAR T cells improves efficacy against solid tumors in mice”.
The study shows that CAR-T cells can deliver oncolytic viruses into solid tumors. The oncolytic virus can then penetrate tumor cells, replicate, burst the tumor cells, and stimulate a powerful immune response.
“This approach allows tumors to be killed by both oncolytic viruses and CAR-T cells,” explained Richard Vile, Ph.D., corresponding author of the paper and co-director of the Gene and Virus Therapy Program of Mayo Clinic Cancer Center. “In addition, when the oncolytic virus is delivered, it turns the tumor into an extremely inflammatory environment that the patient’s own immune system then sees and starts attacking the tumor.”
New Method Cuts Time to Make CAR-T Cells from Three Days to Within 24 Hours
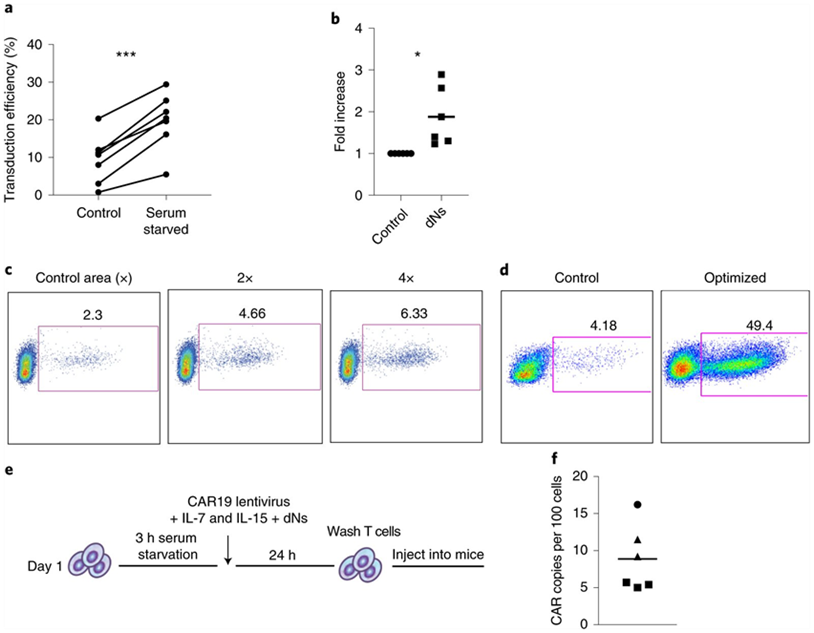
In a new preclinical study, researchers from the Perelman School of Medicine at the University of Pennsylvania have developed a new method that promises to shorten the time it takes to make CAR-T cells to treat cancer patients. Using this approach, they created functional CAR-T cells with enhanced anti-tumor efficacy in as short as 24 hours. These results suggest that the time, materials, and labor required to manufacture CAR-T cells have the potential to be significantly reduced, which is particularly beneficial for patients with rapidly progressive disease and in resource-poor healthcare settings. The research results were recently published in the journal Nature Biomedical Engineering under the title “Rapid manufacturing of non-activated potent CAR T cells”. The corresponding authors of the paper are Dr. Michael C. Milone and Dr. Saba Ghassemi, researchers at the Center for Cellular Immunotherapy at the Perelman School of Medicine at the University of Pennsylvania.
“While traditional manufacturing methods for generating CAR-T cells take days to weeks, there is still a great need for patients with blood cancers such as leukemia to reduce the time and cost to generate these complex therapies,” Milone said. “Building on our 2018 study, which reduced the standard manufacturing method to three days and now to less than 24 hours, this manufacturing method reported in this new study has the potential to improve the generation of CAR-T cell therapies, benefiting more patients.”
Traditional manufacturing methods require stimulating or activating T cells to induce them to proliferate and expand in number. A key to this new manufacturing method developed by the authors is lentiviral vectors that deliver the CAR gene to T cells. HIV-derived lentiviral vectors are able to transfer genes such as CARs into cells without the initial activation step, but the process is inefficient. Using a genetic modification approach based on knowledge of how HIV naturally infects T cells, they developed a way to overcome the necessity for T cell activation and deliver genes directly to inactivated T cells freshly isolated from blood cell. This shows great benefits: it speeds up the entire manufacturing process, while also maintaining the potency of the T cells. And in this process, the patient will not be infected with HIV.
Reference
1. Leick, Mark B., et al. “Non-cleavable hinge enhances avidity and expansion of CAR-T cells for acute myeloid leukemia.” Cancer Cell 40.5 (2022): 494-508.