All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
At Creative Biolabs, we provide a comprehensive range of products and tailored solutions for every stage of CAR-T therapy development, which are designed to streamline workflows and enhance the efficiency of T-cell engineering, helping to overcome technical hurdles and expedite the research. We prioritize close collaboration with academic institutions, biotech companies, and pharmaceutical firms, delivering both technical expertise and ongoing support throughout the development process. With a strong commitment to quality, efficiency, and adaptability, we are dedicated to enabling the full potential of the next-generation CAR-T therapies.
CAR-T cell therapy is a whole new dimension in cancer treatment where T-cells are repurposed to recognize and destroy cancerous cells more effectively. In this variant of cellular therapy, T-cells are genetically modified to express a chimeric antigen receptor, CAR, on their surface; thus, these receptors enable the T-cells to locate certain types of proteins on cancerous cells. The modified T-cells will seek and bind to cancer cells, prompting an immune response that destroys the malignant cells. CAR-T therapeutics represent the leading edge in modern cancer treatment, offering a hopeful and very personalized approach.
Recent developments in next-generation CAR-T constructs and the discovery of new antigen targets have further enhanced existing technology capabilities. These developments create expectations for novel treatment modalities, especially in domains such as solid tumors, where conventionally, CAR-T therapies have faced severe difficulties. Researchers are investigating new ways of further improving the effectiveness and safety of CAR-T cells, from refining cell engineering methodologies to incorporating state-of-the-art technologies such as CRISPR gene editing. This excitement heralds great advancements in immunotherapy and personalized medicine so that CAR-T therapy might soon become the mainstay of cancer treatment.







Going beyond the classical generations of CAR-T cells, the Smart™ CAR platform is designed to push the boundaries through offering construction services for next-generation CAR designs. Included in these are novel constructs such as Dual CARs, TriCARs, Modular CAR, and Logic-gated CAR. This platform can develop very specific structures that allow the targeting of a tumor cell by more than one antigen or that might introduce novel ways of regulating the activity of CAR through advanced regulatory mechanisms. This allows the creation of more specific and personalized therapies that can help to overcome some of the flaws in conventional CARs, such as tumoral evasion mechanism and off-target toxicities. Learn more about Smart™ CAR Construction.
The mRNA-based CAR cell platform is based on transient expression methodologies, uses safer methodology, and sets a better-controlled methodology for cell therapy. It minimizes the risk of genetic modification in a long-lasting manner because of the non-viral delivery of one or more mRNA molecules into PBMCs or isolated immune cells. Thus, this can be one of the essential benefits related to safety with this platform due to the transient nature of mRNA expression, which means off-target and adverse effects are temporary. Besides, non-viral delivery eliminates the risks of viral vectors, such as insertional mutagenesis, thus making it more flexible and a safer option for the development of CAR-T therapies. It is ideal for those patients whose conditions call for short-term immune modulation that does not need to set in place permanent modifications to their genome. Learn more about mRNA-Based CAR Cell Platform.
CRISPR-based engineering of CAR-T cells offers an efficient and precise method for editing T cells, improving therapeutic efficacy. It uses endogenous gene knockout to make allogenic, universal CAR-T cells, and this increases the accessibility of therapy while reducing demand for patient-specific T cells. The technology can also ablate inhibitory receptors on the CAR-T cells that can enhance their persistence and activity within the tumor microenvironment. The potential to integrate the CAR cassette at specific sites within the genome engendered a controlled expression and minimized the potential for unwanted genetic change. This technology offers a major advantage in that it enables cell therapies to be highly personalized, with the added potential even to overcome immune evasion by tumors. Learn more about CRISPR-edited CAR Cell Technology.
Explore our comprehensive suite of technology platforms for more detailed insights and tailored solutions.
Creative Biolabs provided custom-designed chimeric antigen receptor (CAR) constructs essential for customers’ study.
The CD19 CAR consists of an anti-CD19 scFv, CD8 hinge and transmembrane region, 4-1BB intracellular region and CD3ζ intracellular region. The CAR was combined with EGFP by a T2A sequence.
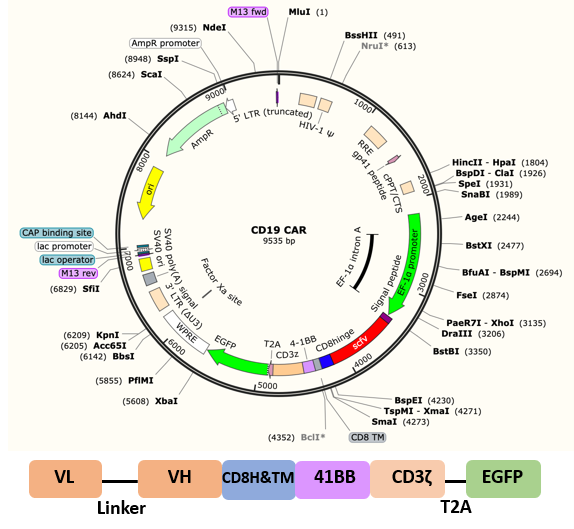 Fig. 1 Map and schematic of CD19 CAR lentiviral plasmid.
Fig. 1 Map and schematic of CD19 CAR lentiviral plasmid.
Creative Biolabs professionally performs multiple evaluations on the post-transfection CAR-T cells to verify the superior function.
Activated T cells were transduced with the lentivirus carrying the anti-CD19 CAR construct in CTS AIM V SFM medium (containing 5% SR and 200 IU/mL IL2). The lentivirus was added at a multiplicity of infection (MOI) of 3-5. The percentage of CAR-positive cells after enrichment and expansion (day 7-10) was determined using flow cytometry (27.63% for the first batch; 61.57% for the second batch). Analysis of CAR-T showed 30-40 fold expansion.
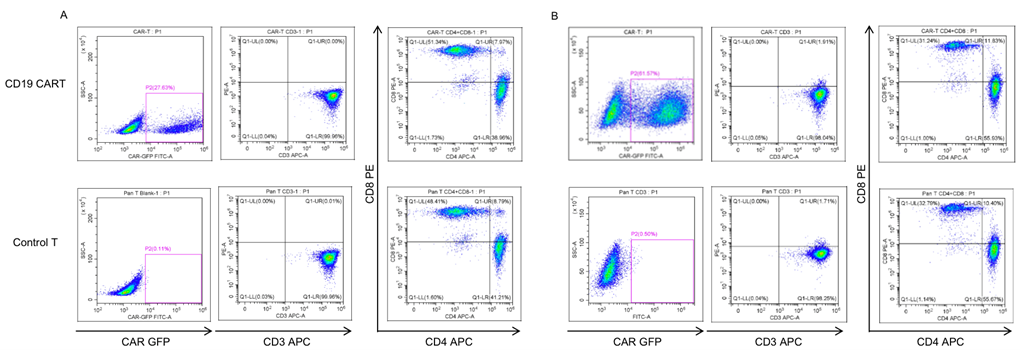 Fig. 2 Characterization of first batch primary CART (A) and second batch primary CART (B).
Fig. 2 Characterization of first batch primary CART (A) and second batch primary CART (B).
The cell viability was analyzed for CART cells after thawing. The average cell recovery after thawing was 70%. 2-3 days after thawing, the CART cells were characterized using FACS. Cryopreservation did not affect the frequency of CAR+ T cells, CD3+ T cells or the CD4+/CD8+ subpopulation composition.
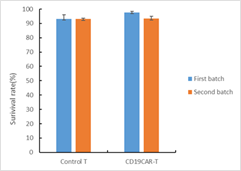 Fig. 3 Cell viability analysis of frozen CART cells after thawing.
Fig. 3 Cell viability analysis of frozen CART cells after thawing.
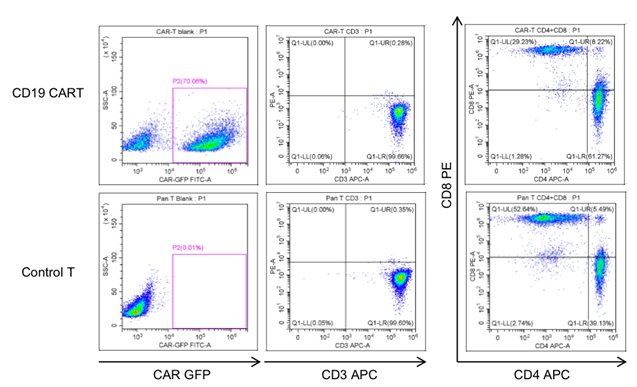 Fig. 4 Characterization of frozen CART cells after thawing.
Fig. 4 Characterization of frozen CART cells after thawing.
The killing of CD19+ CART cells against K562-CD19+ target cells were determined by using FACS. The target cells were co-cultured with CD19 CART cells and control T cells at effector to target (E:T) ratio (2:1).
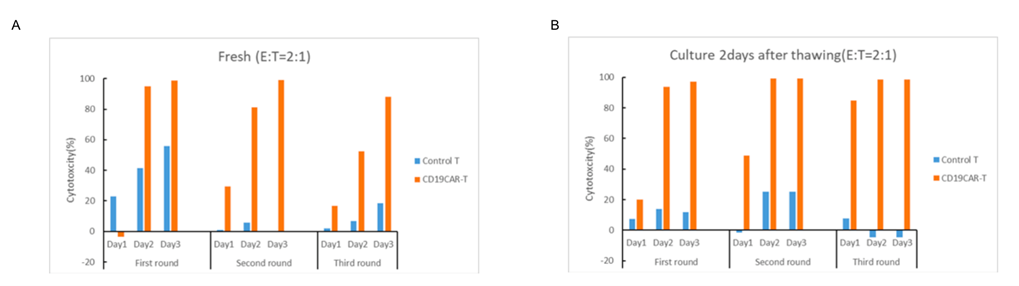 Fig. 5 In vitro cytotoxicity assay of fresh CART and frozen CART cells after thawing.
Fig. 5 In vitro cytotoxicity assay of fresh CART and frozen CART cells after thawing.
At Creative Biolabs, our role is to offer highly specialized, fully customized services for the development of CAR-T cell therapy. Unlike standardized solutions, we make sure the services are precisely designed to identify with the distinctive demand of each client's project. We offer one-stop solutions that assuredly cover the whole spectrum of CAR-T therapy development. The comprehensive approach ensures that every minute detail in the process is seamlessly woven into achieving the highest quality and efficiency toward the advancement of therapeutic development.
One of our key strengths is flexibility and diversity in the design of CARs. We have competence in constructing not only the classical but also the novel and specialized CAR designs; thus, each client has a choice as to which will be most appropriate to fit their needs. We further simplify this process by offering an easy-to-use, completely web-based interface that will enable clients to submit their custom CAR designs with just a few clicks, while our team takes care of technicalities with speed and precision. Innovative approach for eased design, development, and testing of custom CAR-T therapies by the researchers themselves: greatly reducing timelines to reach clinical application.
Our services rely on a team of high-profile, experienced cell engineering professionals. We can offer unmatched expertise at any stage of the project - from conceptual design to complex preclinical studies and even through clinical trials. The broad background ensures that our customers will receive the most innovative solutions fully supported by the latest scientific findings. By leveraging our experience, every project benefits from a mix of in-depth knowledge and practical experience with a commitment to excellence for better results.
By partnering with Creative Biolabs, clients are afforded not only a comprehensive suite of services but also strategic and technical support to successfully navigate the complexities of CAR-T cell therapy development. The commitment to innovation, flexibility, and customer-oriented service ensures that every project reaches its full potential. With Creative Biolabs, you are choosing a partner as invested in the success of your research and clinical goals as you-who has the required tools and expertise for the transformation of bold research into life-changing therapies for cancer.
Use the resources in our library to help you understand your options and make critical decisions for your study.
Chimeric antigen receptor (CAR) can combine the extracellular antigen recognition domain from antibodies with the immune cell signaling domain to redirect T cell specificity and induce potent antitumor activity. CAR is an artificial transmembrane receptor that connects the extracellular antigen recognition domain, hinge domain (HD), transmembrane domain (TMD), and intracellular signal transduction domain in series.
CAR-T cell therapies revolutionize cancer treatment by harnessing the power of a patient's immune cells. Engineered with chimeric antigen receptors (CARs), these cells effectively target and destroy cancer cells, offering a personalized and potent approach. This groundbreaking immunotherapy has shown remarkable success in treating certain blood cancers, providing hope for patients who may not respond to traditional treatments. CAR-T cell therapies mark a significant stride towards precision medicine, ushering in a new era in oncology with the potential to transform the landscape of cancer care.
Based on the significant roles of T cells in the immune system, many small-molecule drugs targeting T cells and T-cell based immunotherapies have been developed for the treatment of intractable diseases including autoimmune diseases and cancer. T cell-based immunotherapies mainly utilize the mechanisms of T cell-mediated immune responses and the effects of some other immune cells such as dendritic cells (DCs), natural killer (NK) cells, and macrophages.
Adoptive cell transfer (ACT) of engineered T cells is a cutting-edge therapeutic approach revolutionizing cancer treatment. This innovative method involves modifying T cells, a key component of the immune system, to enhance their ability to target and eliminate cancer cells. By introducing genetically engineered T cells into patients, researchers aim to bolster the immune response against cancer, offering a personalized and potentially curative treatment option. This groundbreaking technology holds promise for addressing various malignancies and represents a significant stride towards more effective and precise cancer therapies.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION