Treating prostate cancer with immunotherapy remains challenging. However, researchers at City of Hope have developed a chimeric antigen receptor (CAR) T cell (CAR-T) therapy, and the initial results of the first-in-human Phase I trial indicate that advanced prostate cancer patients can safely receive this cellular immunotherapy with promising therapeutic activity. The findings were published in the June 2024 issue of Nature Medicine, titled “PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial.”
This clinical trial treated 14 patients with metastatic castration-resistant prostate cancer (mCRPC) positive for prostate stem cell antigen (PSCA). These patients’ cancer cells had spread beyond the prostate and no longer responded to hormone therapy. In the United States, over 34,000 men die annually from this type of prostate cancer.
Dr. Saul Priceman, co-corresponding author of the study and Associate Professor in the Department of Hematology and Hematopoietic Cell Transplantation at City of Hope, and his team developed CAR-T cells targeting PSCA, which is highly expressed in prostate cancer. This therapy involves extracting immune cells (T cells) from the patient’s blood and reprogramming them in the laboratory with CAR to recognize and attack the PSCA protein on cancer cells. These reprogrammed CAR-T cells are then infused back into the patient to eliminate the cancer cells.
Dr. Tanya Dorff, co-corresponding author and first author of the paper, and Professor in the Department of Medical Oncology and Therapeutics Research at City of Hope, stated, “Prostate cancer is known as an immune desert – its tumor microenvironment is difficult to treat with immunotherapy because there are not many T cells in the tumor. Something very potent is needed to overcome this. Our study shows that City of Hope’s CAR-T cell therapy for prostate cancer may be a step closer to that goal.”
Priceman added, “The primary finding of our clinical trial is that PSCA-targeted CAR-T cells are safe and indeed effective against mCRPC. This provides an opportunity to continue developing this cellular immunotherapy for these patients, who currently have no other effective treatment options.”
The trial aimed to assess the safety and dose-limiting toxicity (side effects that limit the treatment dose) of the therapy, as well as preliminary data on its efficacy for patients. The clinical trial results are as follows:
1. Patients who did not undergo lymphodepletion chemotherapy beforehand received a single infusion of 100 million CAR-T cells. Lymphodepletion chemotherapy is a standard method in blood cancer treatment to enhance CAR-T cell efficacy. Since this was the first human CAR-T cell trial, it was necessary to assess the safety of using CAR-T cells alone.
2. Dose-limiting toxicities such as cystitis or bladder irritation were observed at the same CAR-T cell dose and lymphodepletion. Dorff explained that PSCA is also present in the bladder, so CAR-T cells likely attacked bladder cells, causing inflammation. The researchers subsequently added a new cohort to the clinical study using reduced lymphodepletion to mitigate this toxicity.
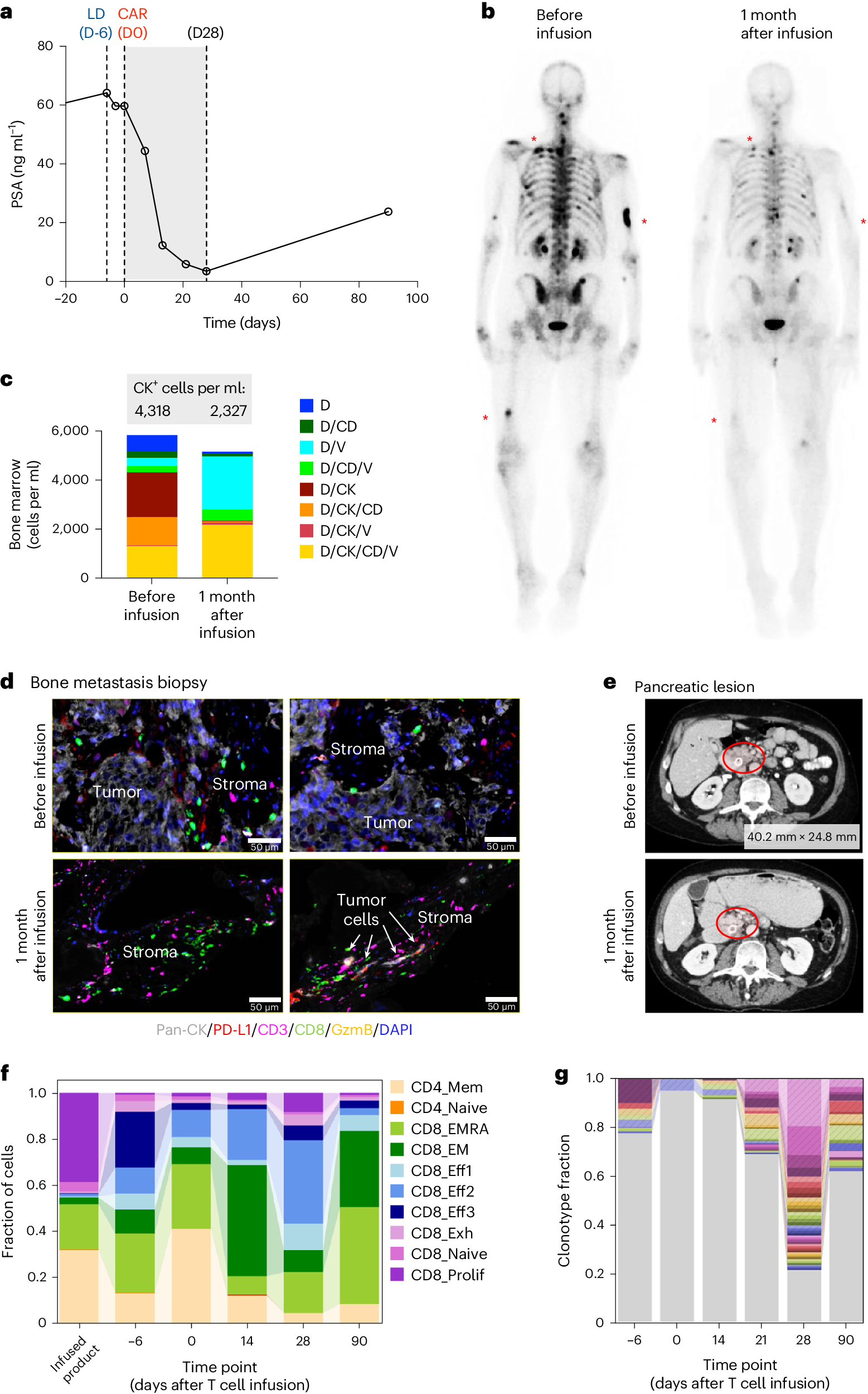
3. Four out of 14 patients showed a decrease in prostate-specific antigen (PSA) levels, a serum marker of disease progression in prostate cancer patients. One patient had a significant PSA level drop. Imaging results indicated a treatment response in some patients.
4. Five out of 14 patients experienced mild to moderate cytokine release syndrome (CRS), a common side effect of CAR-T cell therapy caused by the rapid release of cytokines into the blood by immune cells. CRS is a treatable side effect.
5. CAR-T cells did not maintain high levels after the 28-day monitoring period, limiting the therapy’s effectiveness. This is a common challenge in the CAR-T cell field for solid tumors, and the researchers plan to address this in follow-up clinical trials currently open for recruitment at City of Hope.
One patient who had undergone multiple treatments responded well to this CAR-T cell therapy. His PSA level decreased by 95%, and there was a reduction in cancer in his bones and soft tissue. This positive response lasted about eight months.
Dorff remarked, “The results for this patient are very encouraging, and we are very grateful to him and other patients and their families for participating in our research. We hope to continue using this therapy, increasing the number of CAR-T cells, and carefully monitoring for any health issues, as we believe this can enhance the therapy’s effectiveness.”
Creative Biolabs masters the most advanced CAR/TCR technology. With state-of-art TCR development platforms and advanced technologies, Creative Biolabs is capable of offering a broad range of CAR-T therapy development services, including TCR engineered T cell biomarker identification and selection, design, construction, and analysis. To support the development of PSCA-targeted CAR therapy, Creative Biolabs has developed a comprehensive series of PSCA molecular-related products, such as CAR-T/CAR-NK/CAR-Macrophage Vectors and Cells, AbTCR/TriCAR Vectors, CAR Viral Particles, TCR-Like CAR, CAR Animal Cells, CAR mRNA Lipid Nanoparticles
Reference
1. Dorff, Tanya B., et al. “PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: a phase 1 trial.” Nature Medicine (2024): 1-9.