T cells expressing chimeric antigen receptors (CAR-T) are one of the major breakthroughs in the field of cancer treatment in recent years, and they have achieved excellent efficacy in the treatment of blood cancers. Chimeric antigen receptors (CAR) are like equipping T cells with a GPS navigation, allowing T cells to quickly locate tumor cells that are good at camouflage, so as to find and kill them. So far, three CAR-T therapies targeting CD19 have been successfully marketed globally for the treatment of B-cell malignancies.
CAR-T therapy has shown great strength in hematological malignancies, but there are still many obstacles in the treatment of solid tumors. The main reason is that there are many difficulties in the cell therapy of solid tumors, such as the heterogeneity of different types of solid tumors, the lack of unique tumor-associated antigens as CAR-T targets, the inability of T cells to effectively home to the tumor site, insufficient persistence of CAR-T cells and the complex microenvironment within the tumor which has a suppressive effect on immunity.
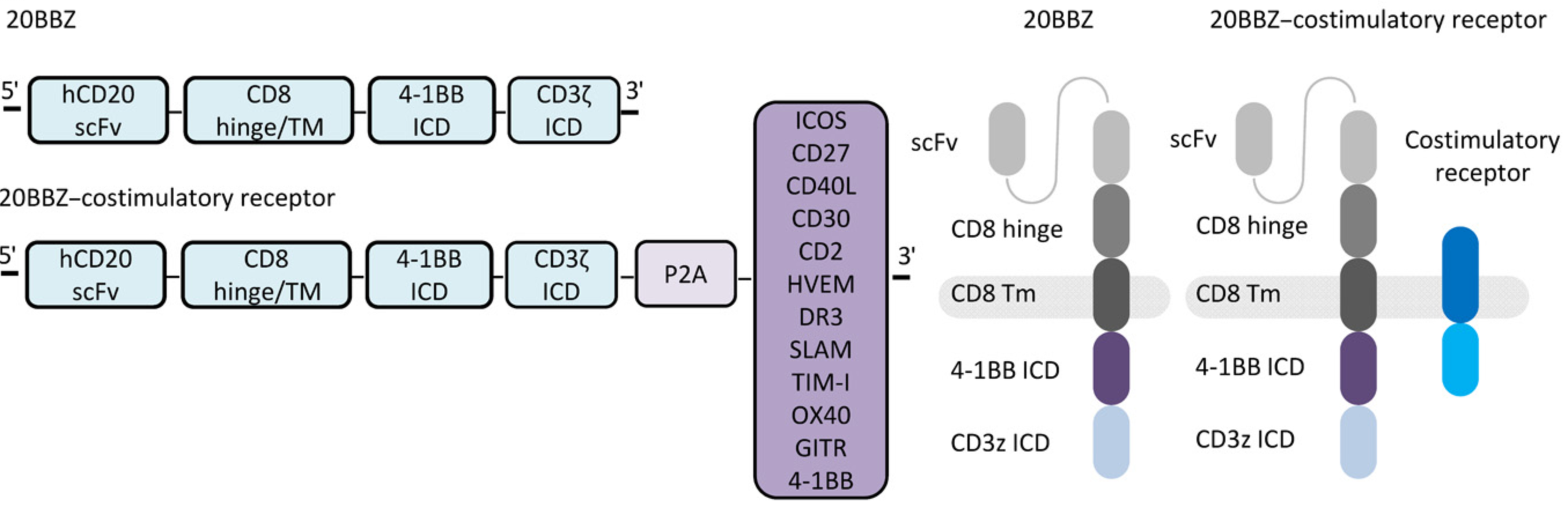
Generally, T cell activation requires at least two signals—in addition to the first signal recognizing MHC-peptide compounds, various signal stimuli during the recognition process are also necessary. To solve the problem of CAR-T cell treatment of solid tumors, scientists have introduced new costimulatory domains, cytokine genes, and cytokine receptors into T cells to enhance the persistence and anti-tumor activity of CAR-T cells. Recently, the team of Associate Professor Xuanming Yang of Shanghai Jiao Tong University published a research paper in the journal Science Translational Medicine that OX40 costimulatory signal can enhance the anti-tumor activity of CAR-T.
At present, most costimulatory signals are transduced together with T cell receptor signals in the process of T cell activation, but in this research, the researchers designed a kind of costimulatory signals independent of T cell receptors, which can be activated and stimulates T cells. Researchers screened 12 costimulatory receptors, including ICOS, CD27, CD40L, and CD30, and found that OX40 has the most significant effect on CAR-T amplification.
OX40 is one of the important T cell costimulatory signals and is a member of the tumor necrosis factor (TNF) receptor superfamily. The OX40 ligand OX40L (also known as CD252) combines with 3 molecules of OX40 protein in the form of a trimer to form a hexamer complex, which activates downstream signaling pathways such as NF-κB, PI3K, and AKT. The continuous activation of these downstream signals can stimulate the production of cytokines, prolong the survival time of T cells, inhibit the differentiation and activity of regulatory T cells (Tregs), and enhance the killing ability of effector T cells.
In the experiment, the researchers also found that OX40 can reduce the apoptosis of CAR-T cells by up-regulating the expression of genes encoding Bcl-2 family members, and enhance the cells by increasing the activation of NF-κB, MAPK and PI3K-AKT signaling pathways proliferation. In addition, the OX40 signal not only enhances the cytotoxicity of CAR-T cells, but also reduces the biomarkers in failure, so that the function of CAR-T cells can be maintained in the immunosuppressed tumor microenvironment.
Refering to in vivo experiments, the OX40 costimulatory signal significantly enhances the anti-tumor activity of CAR-T cells and has a significant inhibitory effect on metastatic tumors, indicating that the OX40 costimulatory signal is expected to be used in the treatment of solid tumors. Based on the method of this research, the autologous 20BBZ-OX40 CAR-T therapy has entered the phase I clinical trial. And the therapy currently shows good tolerability and efficacy in patients suffering B-cell lymphoma.
In recent years, scientists have made considerable efforts to develop new methods to overcome the obstacles of solid tumors and adopted optimized strategies for CAR-T therapy for these specific indications. It’s expected that the true capabilities of CAR-T in the treatment of solid tumors can be explored as more and more technological and clinical breakthroughs occur.
Reference
Zhang, Huihui, et al. “A chimeric antigen receptor with antigen-independent OX40 signaling mediates potent antitumor activity.” Science translational medicine 13.578 (2021): eaba7308.