Activated T cells with certain surface markers, such as checkpoint inhibitor proteins, are regulated by another type of immune system cell—natural killer (NK) cells. In this way, the body likely suppresses destructive immune responses. Researchers from the German Cancer Research Center and Mannheim University Medical Center have now discovered that NK cells can impair the effectiveness of immune checkpoint inhibitor (ICI) cancer therapies in this manner. They may also contribute to the rapid reduction of therapeutic CAR-T cells. Interventions targeting this mechanism could potentially enhance the efficacy of these cellular immunotherapies for cancer. The findings were published in the May 2024 issue of Science Immunology under the title “The immunoglobulin superfamily ligand B7H6 subjects T cell responses to NK cell surveillance”.
T cells in the immune system play a critical role in combating viral infections and tumor cells. However, they can also attack healthy tissues in autoimmune reactions, potentially with fatal consequences. Therefore, the body tightly controls the activity of T cells.
A myriad of molecules and messengers are involved in the highly complex regulation of T cell activity. Recently, scientists have discovered that another type of immune cell is also involved in controlling T cell activity. NK cells are part of innate immunity, acting as a rapid response force capable of swiftly detecting and eliminating infected or malignant cells.
Michael Platten, co-corresponding author and head of the department at the German Cancer Research Center, stated, “Previous studies have shown that NK cells can kill activated T cells, thereby limiting their proliferation. However, it was not known what characteristics of T cells make them targets for NK cell action.”
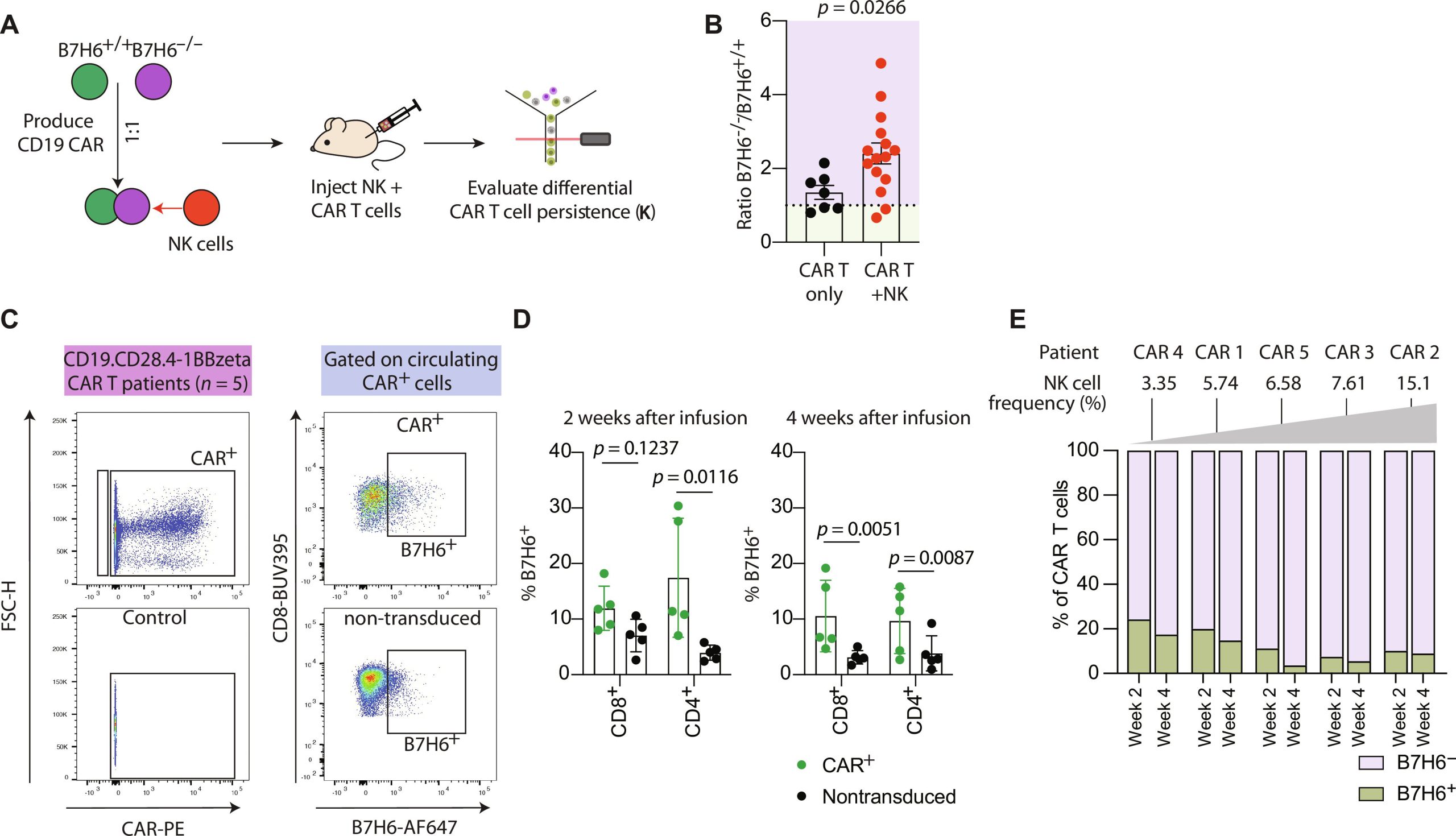
In this new study, the team led by Platten screened activated T cells from healthy donors and identified the protein B7H6 as a recognition molecule targeted by NK cells. Activated T cells from patients with autoimmune diseases, cancer, or viral infections showed significant surface expression of B7H6. Co-culture experiments conducted in petri dishes demonstrated that NK cells recognize activated T cells through the expression of B7H6. In contrast, T cells with the B7H6 gene disrupted by CRISPR-Cas did not undergo lethal attack by NK cells.
Michael Kilian, co-corresponding author and first author of the paper, explained, “NK cells eliminate T cells through mechanisms intrinsic to T cells themselves. Activated T cells temporarily identify themselves as targets for NK-induced cell lysis,” and added, “This could potentially limit excessive activation and expansion of T cells as a control mechanism against destructive immune responses.”
Platten elaborated, “We now know that certain immune checkpoint molecules can either diminish or enhance T cell activation, thus modulating the immune response process. B7H6 can now be classified as another inhibitory immune checkpoint on the surface of T cells.”
Some widely used cancer therapies utilize immune checkpoint inhibitors (ICI) that target specific inhibitory immune checkpoint molecules. They work by releasing the immune system’s brakes to activate immune responses against tumors. Could the clearance of tumor-reactive T cells mediated by B7H6 counteract the efficacy of ICI cancer immunotherapies? The authors tested tissue samples from esophageal cancer patients who had undergone ICI therapy. Patients who did not respond to ICI treatment had more NK cells in their tumor tissues and shorter progression-free survival.
Cellular immunotherapy is becoming increasingly important in cancer medicine. For example, certain forms of blood cancers now often use CAR-T cells, which carry customized receptors targeting cancer cells. However, the success of these therapies is often limited by the rapid depletion of therapeutic CAR-T cells in patients.
These therapeutic CAR-T cells also express B7H6 on their cell surfaces. Could NK cells lead to their rapid depletion following treatment initiation? Experiments in humanized mouse models suggest this possibility: the addition of NK cells during CAR-T cell therapy for leukemia led to a reduction in therapeutic CAR-T cell numbers and an increase in tumor burden.
Platten explained, “NK cell control of T cells could interfere with various forms of cancer immunotherapy. By specifically intervening in this process, it may be possible in the future to modulate immune responses of T cells.” With the help of CRISPR-Cas gene scissors, they now hope to collaborate with the Department of Hematology and Oncology at Heidelberg University Hospital in clinical trials to protect CAR-T cells from NK cell elimination, thereby enhancing the efficacy of cellular immunotherapy.
Creative Biolabs masters the most advanced CAR/TCR technology. With state-of-art TCR development platforms and advanced technologies, Creative Biolabs is capable of offering a broad range of CAR-NK therapy development services, including biomarker identification and selection, CAR-NK vector design and construction, and in vitro/in vivo assays.
Reference
1. Kilian, Michael, et al. “The immunoglobulin superfamily ligand B7H6 subjects T cell responses to NK cell surveillance.” Science Immunology 9.95 (2024): eadj7970.