On February 16, Iveric Bio announced in an announcement that the New Drug Application (NDA) for the complement C5 inhibitor Zimura (avacincaptad pegol, ACP) for the treatment of age-related macular degeneration (AMD) secondary to geographic atrophy (GA) was granted priority review by the FDA with a PDUFA date of August 19, 2023. This is the second GA therapy and the fourth complement C5-targeted therapy to be filed for marketing worldwide.
GA is an advanced manifestation of AMD, resulting from the loss of photoreceptors, the retinal pigment epithelium (RPE), and underlying choroidal capillaries, which can lead to a progressive and irreversible loss of visual function. This progressive disease can severely diminish the quality of life of patients as it slowly invades the central retinal recess, which is critical for central vision, over a period of 2.5 years. No GA therapy is currently approved for marketing.
Zimura, a polyethylene glycolated RNA aptamer designed to inhibit the complement C5 protein, was first developed by Archemix, and in August 2007, Iveric entered into a licensing agreement with Archemix to acquire worldwide development and commercialization rights for Zimura as an ophthalmic drug. Researchers believe that overactivation of the complement system and the C5 protein play a key role in the development of scarring and vision loss associated with AMD secondary to GA. By blocking C5 protein activity, Zimura may reduce the activity of the complement system that causes retinal cell degeneration and may slow the progression of GA. The product was granted Fast Track Approvals by the FDA in April 2020.
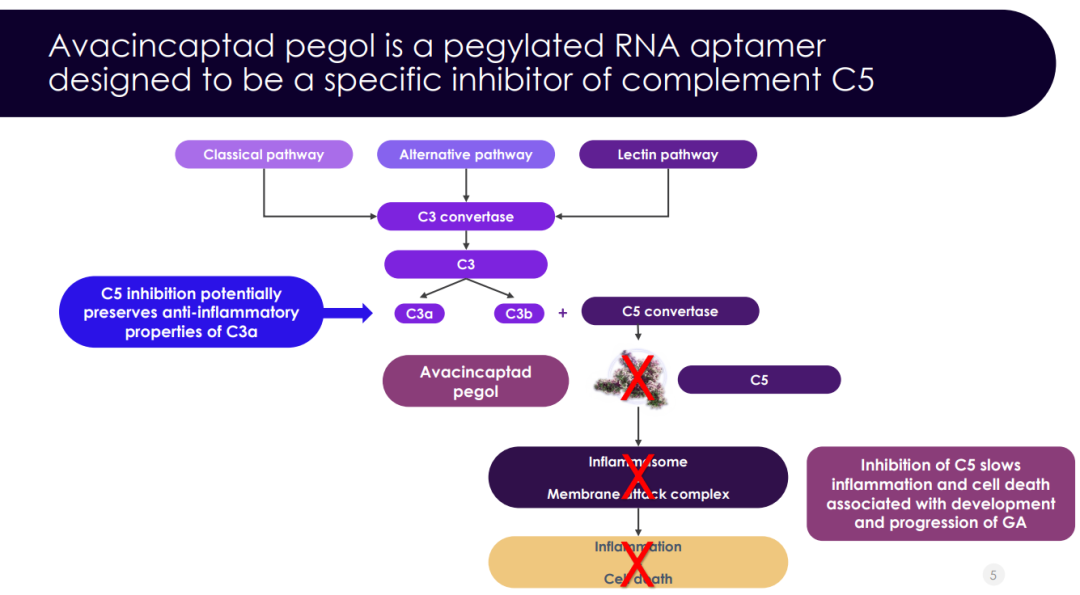
This NDA is based on the positive findings of two phase III clinical trials (GATHER1 and GATHER2). 286 and 448 patients were enrolled in the GATHER1 and GATHER2 studies, respectively, to evaluate the efficacy and safety of Zimura for the treatment of patients with GA secondary to AMD. The co-primary endpoint was the mean rate of change in GA lesion area at month 12.
Results from both studies showed that patients in the Zimura group had a significantly lower mean growth rate of GA lesion areas compared to the placebo group.
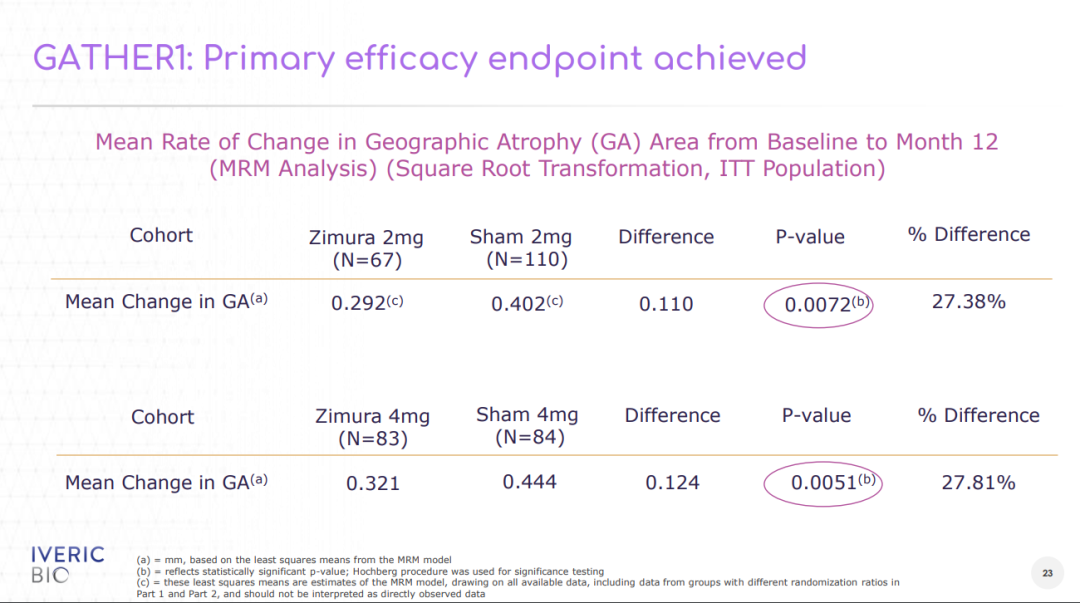
In the GATHER1 study, the mean growth rate of GA lesion area was reduced by 27.38% (p=0.0072) in the Zimura (2 mg) group and by 27.81% (p=0.0051) in the Zimura (4 mg) group of patients compared to the placebo group.
In the GATHER2 study, patients in the Zimura (2 mg) group had a 14.3% lower mean growth rate of GA lesion area compared to the placebo group (p=0.0064). At month 12, Iveric Bio analyzed the mean change in patients’ best-corrected visual acuity (BCVA) and low luminance best-corrected visual acuity (LL BCVA). The data showed that patients in the Zimura group had better BCVA improvement than the placebo group and were consistent with the GATHER1 study, but there was no significant improvement in patients’ LL BCVA.

Prior to Zimura, the first GA therapy, pegcetacoplan (developed in collaboration with Apellis and Swedish Orphan Biovitrum), received priority review by the FDA in June 2022, with a PDUFA date of February this year.
Disclaimers: Creative Biolabs is intended to encourage communication and learning among colleagues in the pharmaceutical industry. This article is not meant for commercial use. We respect original works. The information in this article is for general reference only and should not be used for direct decision-making purposes.
