With the advent of recombinant DNA technology, many antibody fragments have been developed. Among them, camelid heavy chain variable domains (VHHs) and single chain variable domains (ScFv) are the most favored variable domains. Camelid single domain antibodies (sdAbs) are being extensively investigated as an alternative due to their superior chemical and physical properties such as higher solubility, stability, smaller size, and lower production cost.
Structures of Single Domain Antibodies and ScFv
ScFv is an engineered form of Fv in which instead of a disulfide bond, two variable domains are linked together by a flexible linker. The length and amino acid composition of this linker play an important role in the correct folding of the protein. It is usually 10-25 amino acids in length, in which the Glu-Lys stretch increases solubility, and the Gly-Ser stretch increases flexibility. Each of the two variable domains in ScFv has three complementarity determining regions (CDRs) linked to the framework regions (FRs). CDRs are responsible for antigen binding, and their structure is complementary to the epitope, while the framework regions (FRs) act as scaffolds without much variability compared with CDRs. The contribution of each CDR in antigen binding is different. For example, HCDR3 in the heavy chain contributes 29% to binding specificity, whereas LCDR2 only contributes 4%.
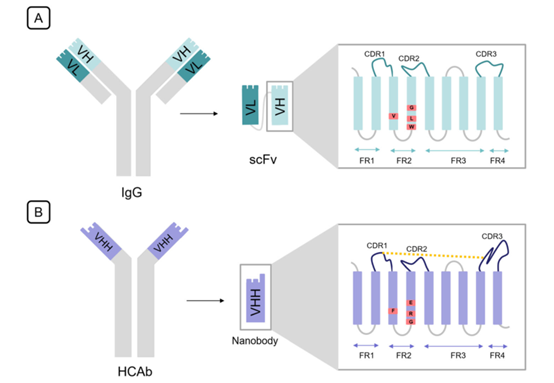
Fig. 1 The differences between scFv and single domain antibody in structure. (Asaadi, Y., et al., 2021)
A hypothesis whether a single heavy chain could retain the binding affinity of the parental Ab was raised due to the higher contribution of VH in antigen binding. After some research, it was found that some of their unfavorable properties, such as low affinity, poor solubility, and higher production cost hindered their development.
The discovery of heavy chain-only antibodies (HCAbs) in camels and immunoglobulin neoantigen receptors (IgNARs) in cartilaginous fish represents a new starting point for the development of single domain antibodies. The antigen-binding domain (VHH and V-NAR) of these specific immunoglobulins is a high-affinity V-type domain that has evolved without the drawbacks of the conventional single heavy chain segment of the past. These excellent properties originate from major adjustments in sequence and structure. VHH fragments are used more frequently due to easier handling, stronger antibody responses, and higher recombinant expression yields than shark V-NARs for camelid single domain antibodies.
Similar to VH, camelid VHH consists of nine β-bands that form a typical IgV fold, but deletion of VL results in significant differences between these two fragments, especially in FR2 and hypervariable loops. In a traditional VH region, FR2 consists of four highly conserved hydrophobic amino acids (Val37, Gly44, Leu45, and Trp47), which together with Gln39, Gly44, Tyr91, and Trp103 form a conserved hydrophobic interface to facilitate VL connection. In single domain antibodies absent VL, these four hydrophobic residues were replaced by more hydrophilic amino acids (Phe37, Glu44, Arg45, and Gly47) to avoid exposure of such a large hydrophobic region to solvent. In addition to this substitution, residues near this interface rotated their side chains without deforming the C-α backbone, thereby increasing the hydrophilicity of the VHH surface. Furthermore, the CDR3 domain of VHH folds over this interface to protect amino acids previously covered by VL. These changes illustrate the higher solubility of VHHs than ScFv and conventional single heavy chain VHs.
In VHH, the expansion of loops in the hypervariable region compensates for the loss of diversity in the 3 VL CDRs and VH-VL combinations. The extension of CDR1 and CDR3 provides a 600-800A2 antigen interaction surface provided by six loops from the VH-VL domain. Furthermore, elongated CDR1 imprinted with mutational hotspots in the VHH germline compensates for VL partner variability. While the extended CDR3 can reach epitopes that are barely accessible to conventional antibodies, the enlarged loop implies greater flexibility, hindering the entropic binding of antigens. To solve this problem, VHHs have evolved an additional disulfide bond towards CDR1, CDR2, or FR2. All of these structural features increase epitope diversity and allow for a wide variety of geometric loop structures that deviate radically from the canonical loop structures defined by conventional antibodies and contribute to the orientation of the CDR3 toward the antigen.
Physicochemical Properties of Single Domain Antibody and ScFv
Due to significant structural differences, ScFvs and single-domain antibodies exhibit different properties in vitro and in vivo.
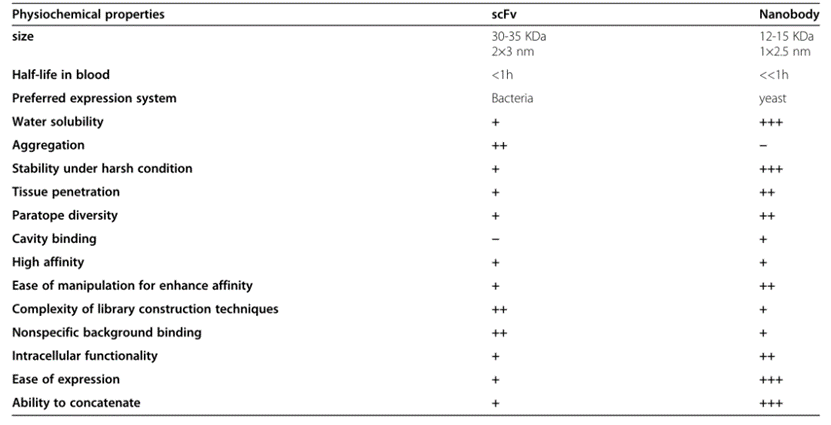
Fig. 2 A comparison between scFv and single domain antibody insdAb properties. (Asaadi, Y., et al., 2021)
Size
First and foremost, the two fragments differ significantly in size. ScFvs are almost twice the size of single domain antibodies and weigh around 30 kDa. The smaller size facilitates the genetic manipulation of VHH, and the presence of only three antigen-binding loops can easily enhance its inherent antigenic propensity. The smaller size of VHHs also results in their shorter half-life in the blood due to filtration and degradation by the kidneys. This feature may be helpful as it leads to high tissue permeability. But disadvantageously, because their molecular weight is lower than the glomerular filtration cut-off size (65 KDa), there may be problems in some clinical treatments that require prolonged antibody circulation. This limitation has led to the development of half-life extension strategies that combine VHHs with additional molecules. One of the most popular of these involves the addition of stabilizing groups, such as polyethylene glycol (PEG) molecules, which can slow blood clearance. A fusion of long-circulating serum proteins such as albumin or specific binders of albumin can effectively increase the half-life of VHH in blood. Fc fusions can stabilize them in the blood and meanwhile stimulate the immune system to reach the target site. In addition, Fc or albumin fusions make antibody fragments larger and enable FcRn-mediated circulation to increase the half-life in the blood.
Solubility and Stability
As mentioned earlier, in VHH, four highly conserved hydrophobic amino acids were replaced by more hydrophilic residues, resulting in a marked difference in properties between single domain antibodies and ScFv. In ScFv, these four residues (V37, G44, L45, and W47) in FR2 form a hydrophobic interface that facilitates VH-VL linkage. However, on the downside, this hydrophobic region reduces the solubility of ScFvs, causing them to have a high tendency to aggregate. Substitution of polar and minor amino acid residues at this position (F37 or Y37, E44, R45, and G47) makes them more hydrophilic and therefore more soluble than ScFv. Furthermore, this nonpolar-to-polar transition results in the molecular and thermodynamic stability of VHHs compared to ScFvs. Therefore, single domain antibodies are more resistant to chemical denaturants and proteases, and have higher stability under harsh pH or ionic strength. This higher conformational stability of VHH also stems from the presence of additional disulfide bonds, which reduce the possibility of heat-induced aggregation and limit the flexibility of VHHs. Due to the higher stability, they show high refolding efficiency, which means that increasing or decreasing the sample temperature does not affect the single domain antibody conformation, or disengagement and binding of the target without any aggregation or denaturation. This structural rigidity is a clinically preferred attribute, as non-native protein aggregation is a common drawback of antibody therapy which in severe cases can increase immune response.
Immunogenicity
A major disadvantage of scFvs is that they are mostly derived from rodents, since hybridoma technology is only well developed in mice and rats. The sequence homology of mouse VL and VH to the human corresponding regions is only 53% and 51%, respectively. However, single domain antibodies show high sequence similarity to human VH (VH3 gene family), and about 75-90% homology is associated with lower immunogenicity in clinical applications. Therefore, VHH is more humanized. Even after the humanization of the murine scFv, the variable region of the scFv can still elicit an anti-idiotypic response. In addition, engineering scFv to reduce human anti-mouse antibody (HAMA) responses may inactivate injected scFv and reduce its clinical effectiveness, and hypersensitivity reactions may occur upon repeated administration. Furthermore, humanization reduced the binding affinity of these fragments, and CDR grafting may represent novel immunogenic epitopes. In general, the humanization of murine scFv can overcome some but not all these immunogenicity problems.
Affinity
Although ScFvs and single domain antibodies share similar affinities, they display a clear preference for epitopes. Single domain antibodies are more likely to access grooves and clefts on the surface of antigens such as ion channels, viral glycoproteins, or immune synapses, while ScFvs prefer flat and linear epitopes. These differences are due to the longer CDR3 loop in single domain antibodies that allows a highly convex shape to access concave epitopes. Single domain antibodies also exhibit good affinity for planar epitopes, suggesting that these fragments can form various interfacial complexes. Furthermore, single domain antibodies show a lower nonspecific background binding than ScFv.
