In 1993, Professor Hamers-Casterman, an immunologist at the Free University of Brussels in Belgium, and his colleagues discovered a new type of antibody in camel serum, which is different from traditional antibody and consists of only two heavy chains, and was thereby called heavy-chain antibody (HCAb). The variable region of HCAb is named VHH whose molecular weight is only 15 kDa, about 1/10 of that of traditional antibody and about 1/2 of that of antigen binding fragment (scFV, VH-VL).
As a single-domain antibody is encoded by a single gene and has a small molecular weight, it is easy to be genetically engineered and can be polymerized to form multivalent or multi-specific antibody structures through short linking sequences. Single-domain antibodies can be divided into monovalent single-domain antibodies, multivalent/multi-specific single-domain antibodies, fusion single-domain antibodies, and so on.
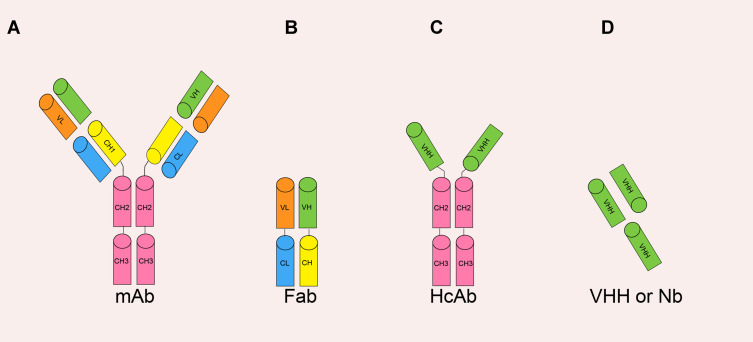
The various antibody formats. (Sun S, et al., 2021)
Monovalent single-domain antibody: an antigen-specific single-domain antibody screened from a single-domain antibody library with specific antigens. A large number of hydrophilic residues on its surface help maintain a strict monomer structure and contribute to binding to its antigen with high specificity and affinity only in this monomer form.
Multivalent/multi-specific single-domain antibody: multivalent antibodies are polymers of monovalent antibodies that recognize the same antigenic epitope and have higher antigen affinity than monovalent antibody. Multi-specific antibodies are polymers of monovalent antibodies that recognize different antigenic epitopes, which can bind different targets, or different epitopes of the same target, and have higher antigen recognition competence than monovalent antibodies.
Fusion single-domain antibody: the monomer characteristics and a relative small molecular weight of single-domain antibody make it is easy to form new fusion molecules with other structures (such as BSA and IgG-Fc) through genetic engineering. In the new fusion molecule, the single-domain antibody binds to its target antigen, and thefusion parts can perform the corresponding functions.
A single domain antibody has only three complementarity-determining regions (CDRs) of heavy chain. To make up for the lack of biological activity caused by light chain CDR shortage, the number of amino acids in CDR3 of single-domain antibodies is added to improve diversity and specificity. In addition, the CDR3 region of single-domain antibodies can form a large exposed protruding ring, so they can reach the antigenic epitopes that traditional antibodies cannot. A cysteine in the convex ring of CDR3 can also form a disulfide bond with the cysteine on CDR1 or FR2 (framework region 2), conducive to a more stable biological structure of the single domain antibody and greatly reducing the energy required for the antibody to bind to the antigen.
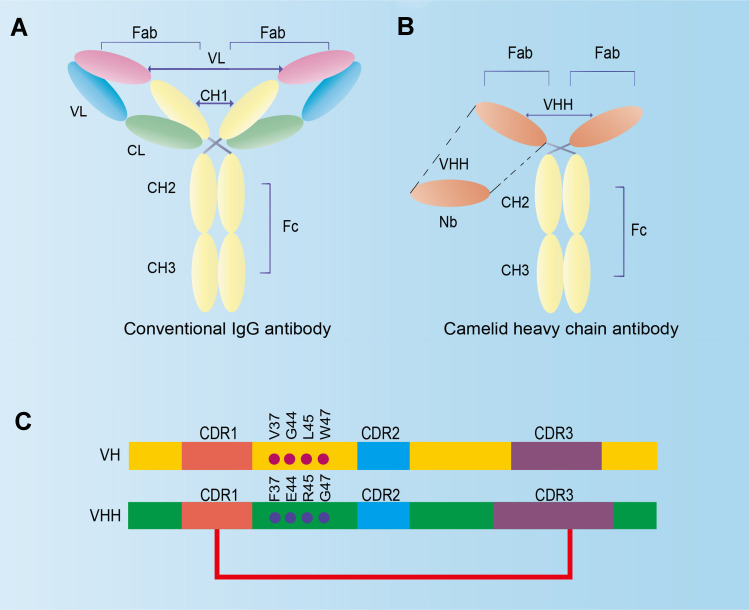
Comparison between traditional IgG antibody and camelid heavy-chain antibody. (Sun S, et al., 2021)
Single domain antibodies have the following advantages:
Stable Physical and Chemical Properties
Single domain antibodies have particularly stable biophysical and biochemical properties, including solubility, heat resistance, and proteolysis resistance. The many hydrophilic amino acids on the CDR3 region of single-domain antibodies make them highly soluble in aqueous solutions and less prone to aggregation.They also has good conformational and thermal stability and can maintain full binding ability after 1 week at 37℃. In addition to heat tolerance, they are also stable under protease, solvent promotion, and extreme pH conditions. The stability enables single-domain antibodies to be administered in different ways like intravenous and oral and inhaled preparations, among which oral and inhaled preparations are difficult to achieve in traditional antibody drugs. If used in clinical diagnosis, its high stability provides great convenience for the detection of small molecules or proteins.
Easy to Produce on a Large Scale
Compared with traditional antibodies with complex structure and high production cost, single-domain antibodies can be expressed in large quantities in the microbial systems (bacteria, yeasts, and fungi), and can be screened quickly by display library, greatly cutting down the production cost.
Low Immunogenicity
The structure of single domain antibody is very close to that of human antibody heavy chain variable region (VH), with as high as 90% genetic homology, which means that the immunogenicity of single domain antibodies is relatively slight. No reports of adverse reactions caused by the immunogenicity of single-domain antibodies has been reported so far. Considering the potential safety risks, it is still necessary to humanize single-domain antibodies during drug preparation. Humanization can be achieved by direct site-directed mutation due to its short gene sequence, much easier than traditional antibodies.
Strong Penetration Capability and Easy to Remove
Monovalent single domain antibody (15 kDa) has better tissue penetration and can reach the tissue that cannot be accessed by traditional antibody (150 kDa). In addition, single domain antibody can effectively penetrate the blood-brain barrier, providing a new method for brain drug delivery. At the same time, single-domain antibody is easily cleared by the kidney and has short half-life that greatly avoids the toxicity and is beneficial to its application in clinical imaging. However, the short half-life limits its use as a therapeutic drug, but can be prolonged by PEGylation, binding or fusion with albumin, and fusion with Fc fragments of traditional antibody.
Capability to Identify Non-Traditional Targets
Benefiting from its small size and special CDR3 structure, single domain antibody can interact with new epitopes that cannot be recognized by traditional antibodies.
Able to Form Polymers
Due to the weak adhesion between single-domain antibody monomers, it is easy to express multiple single-domain antibodies in series by genetic engineering to form polymers. With this property, multivalent antibodies and bispecific antibodies can be obtained to enhance the binding ability of antibodies to antigens. Meanwhile, the half-life of single-domain antibody can also be improved by multimerization or coupling with an antiserum protein, thereby achieving a better therapeutic effect. Single-domain antibodies can fuse with other molecules, so they can be used in combination with other drugs, or used in diagnosis and as an experimental research tool in widespread fields.
