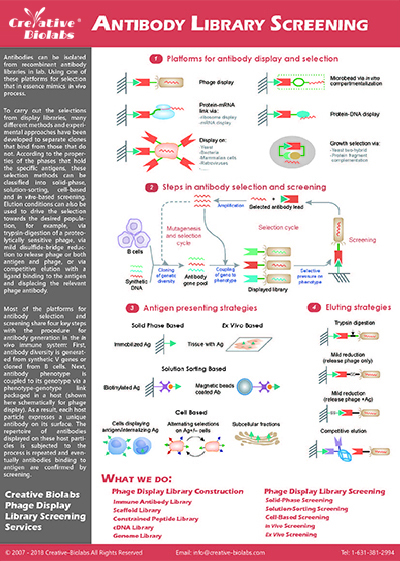
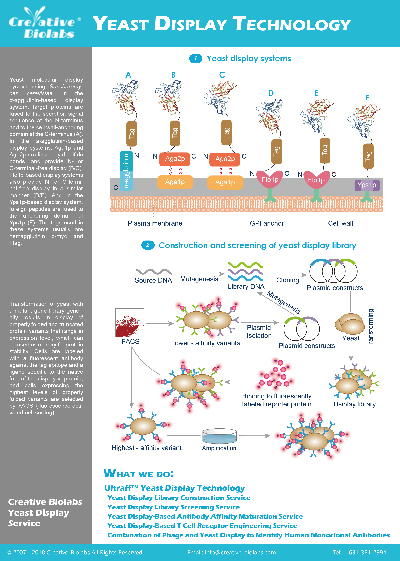
Display System based Single Domain Antibody (SdAb) Discovery Services
Hi-Affi™ Phage Display Platform Yeast Display Platform Immune sdAb Library Naïve sdAb Library Synthetic sdAb Library Case Study Published Data FAQ Resources
Hi-Affi™ Phage Display Platform
Creative Biolabs is recognized as a leading expert in applying advanced Hi-Affi™ Phage Display Platform and our Yeast Surface Display Platform for a broad range of VHH discovery projects. Our Hi-Affi™ platform can generate high-quality
VHH libraries from various sources with maximized diversity and library capacity of up to 1011. Subsequently, our scientists can tailor the best-fit library screening and binder validation procedures to select specific binders with high
affinity, ranging from 108-1012.
Typical Features of Hi-Affi™ Phage Display Platform
-
Proprietary primers designed specifically for the VHH/VNAR domain of corresponding species.
-
Comprehensive phagemid vectors with different detection tags (e.g., His, HA, c-Myc), suitable for various target screenings.
-
Freshly prepared high-quality phagemid vectors, achieving advanced transfection grade for the most sensitive applications.
-
Optimized ligation strategies to increase efficiency, ensure uniformity, and maintain the diversity of constructed phagemid libraries.
-
Nearly 100% correct insertion rate.
-
Outstanding transfection system which can maximize library capacity up to 1011.
-
Comprehensive library screening procedures, specifically designed for the target of interest.
-
Two-step validation strategy, contributing to the most reliable data.
Yeast Surface Display Platform
The yeast surface display system described in Saccharomyces cerevisiae agglutinin is an invaluable tool for generating VHHs. Each VHH can be displayed in high copy numbers (104~105) on the surface of a single cell. Using
this approach, a VHH library with approximately 107~109 clones could be built for further screening and selection of antigen-specific VHH binders.
Our yeast surface display platform allows the use of not only purified proteins but also insoluble proteins and even some unknown targets. Subsequently, VHHs can be efficiently selected using magnetic-activated cell sorting (MACS) and fluorescence-activated
cell sorting (FACS) to yield single-nanomolar affinity VHHs. The yeast surface display platform available at Creative Biolabs is particularly leading and influential due to its unique superiority in MACS and FACS.
Typical Features of Yeast Display Platform
-
Eliminates artifacts due to host expression bias.
-
Includes epitope tags to ensure the selection of full-length VHHs.
-
Features a secretory pathway similar to that of higher eukaryotes, ensuring that only correctly folded proteins are secreted.
-
Provides comprehensive library screening procedures, explicitly designed for insoluble proteins, cells, or unknown targets.
-
Allows quantitative and direct screening with fluorescence-activated cell sorting (FACS).
-
Discriminates VHHs with similar affinities and finds relatively rare VHHs.
Phage and yeast display are powerful and widely used techniques for displaying VHHs on the surface of phages or yeasts and then isolating specific VHHs against the target of interest. Considering the natural differences among various targets, it is crucial
to determine the most suitable source for library construction early in a VHH development project. With our professional scientists who have over ten years of experience in antibody drug discovery, Creative Biolabs has the capacity to tailor the best-fit
source and generate high-quality libraries to lay a solid foundation for your project.
Based on the source, qualified VHH libraries are mainly divided into two categories, immune libraries and non-immune libraries. Non-immune libraries can also be subdivided into naïve libraries and synthetic libraries.
An immune library is constructed from natural infections or immunized donors to obtain antibodies against a particular target. As V genes collected for immune libraries contain hypermutations and have undergone in vivo affinity maturation,
target-specific VHHs with high affinity can be selected if an immune response can be triggered by the target. This approach is widely applicable to various immunogenic targets (e.g., soluble proteins, peptides, DNA, whole cells).
Creative Biolabs has unparalleled capabilities for constructing VHH or VNAR based single domain antibody libraries through immunized camel, llama, alpaca or even shark.
A naïve library is derived from primary B cells of non-immunized donors, in which a large repertoire of naturally rearranged V genes is collected for library construction. Generally, multiple host animals are employed in the VHH repertoire collection,
contributing to a universal library that should not contain bias towards a specific target. In other words, a qualified naïve library is expected to isolate binders against all types of antigenic targets. Typically, the affinity of selected antibodies
from the naïve library is proportional to the size of the library. Due to the lack of in vivo affinity maturation against a specific antigen, an additional affinity maturation process is sometimes required to further increase the affinity
of selected VHHs.
Creative Biolabs can generate a high-quality naïve VHH library for research purposes or industry applications. With our abundant host animal sources and advanced Hi-Affi™ platform, a unique naïve library with a capacity of 1011 can be generated
upon request.
A synthetic library is based on the in silico design and is generated in a controlled manner. Unlike immune or naïve libraries, where the origin of antibody repertoires is amplified from natural sources, the diversity of synthetic libraries
relies on proper design. The ratio of natural to designed components determines whether the library is classified as semi-synthetic or fully synthetic. A typical advantage of a synthetic library is that the composition of CDR regions can be exactly
defined and controlled. It is also a novel option to obtain camelid human single domain antibodies. Similar to the naïve library, synthetic libraries are antigen-independent and can be used for unbiased selection of VHHs against any target.
Creative Biolabs has mature designs and reliable solutions for synthetic VHH library construction. Our scientists provide a series of blueprints for either regular or human VHH libraries, which can fulfill your project demands.
In addition to generating a fully customized VHH library, Creative Biolabs can also provide a cost-effective option for specific VHH discovery with a shorter lead time. In the past ten years, our scientists have generated a series of VHH libraries in-house.
These libraries were preselected based on thermostability and can now serve as a valuable resource for your projects. Some of these premade VHH libraries are even available for licensing, including:
-
CaVHHL-1: Camel Naïve Single Domain Antibody Library
-
CaVHHL-3: Camel Naïve Single Domain Antibody Library
-
CaVHHL-4: Camel Naïve Single Domain Antibody Library
-
LlaVHHL-1: Llama Naïve Single Domain Antibody Library
-
AlpVHHL-1: Alpaca Naïve Single Domain Antibody Library
Creative Biolabs has extensive experience in providing highly customized screening strategies for novel VHH discovery. We have successfully overcome many challenging targets (e.g., membrane proteins, small molecules, and PTM-modified peptides) and selected
specific VHHs. Based on the target properties and your specific project goals, our scientists are pleased to tailor a suitable strategy to achieve your objectives.
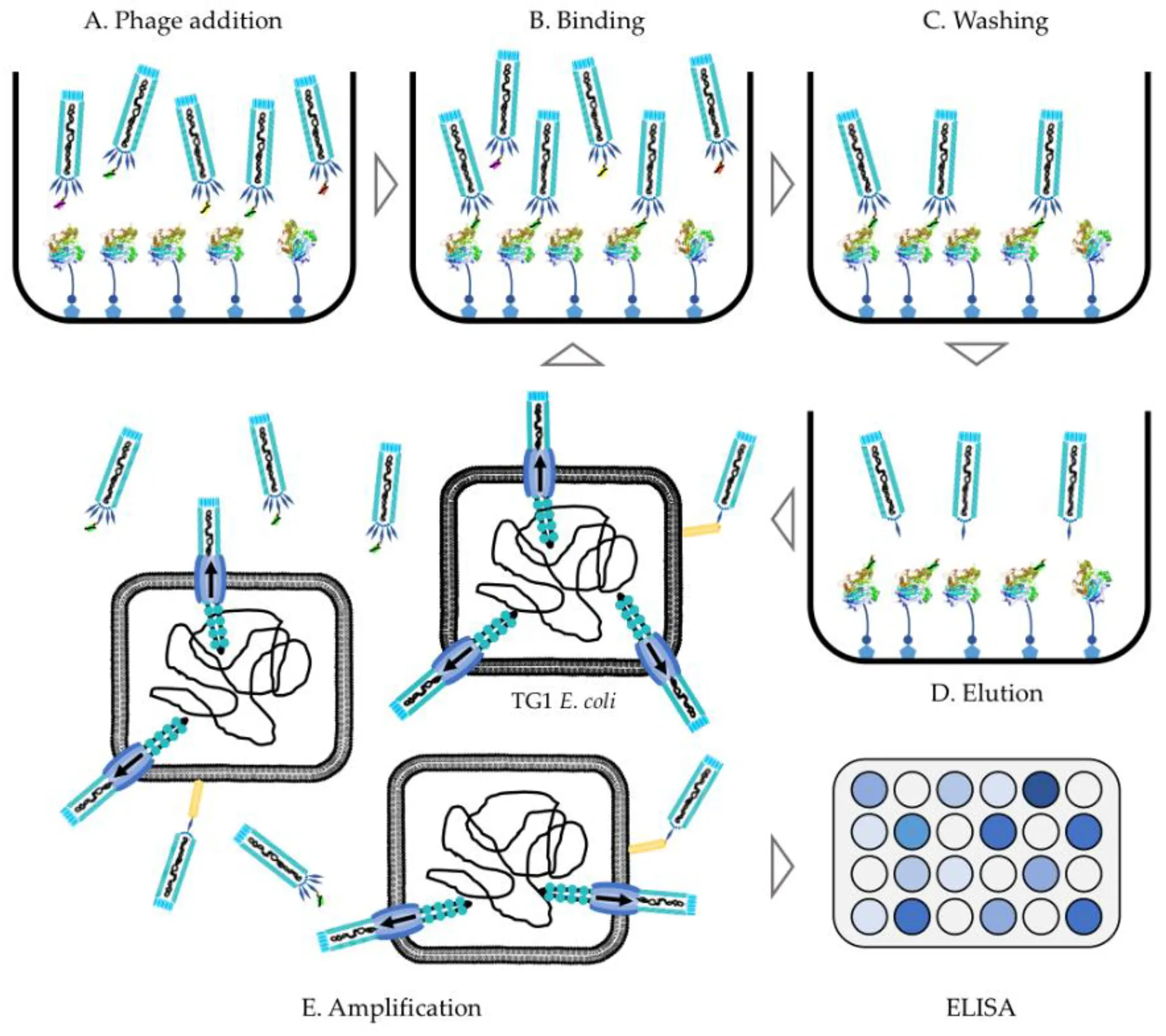
If you are interested in the discovery and development of novel single domain antibodies, please do not hesitate to contact us for more details.
Published Data
-
Anti-CHIKV E2 VHHs Discovery Based on An Alpaca Naïve Phage Display Library
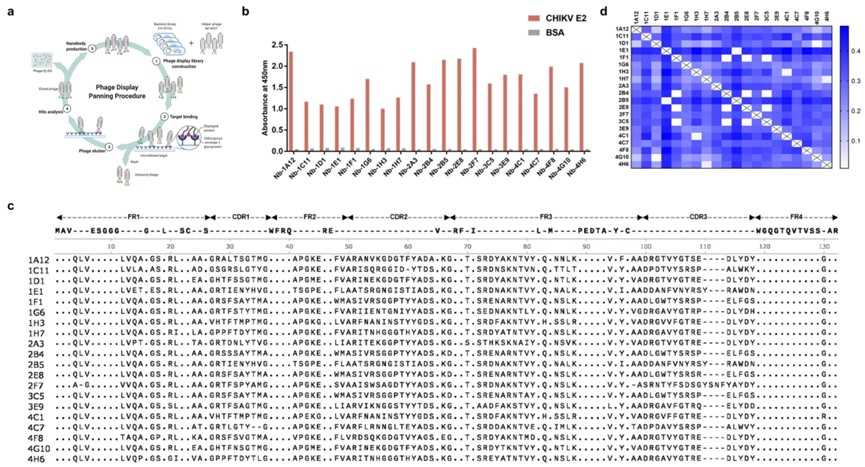 Fig. 1 Identification and Analysis of CHIKV E2-targeting VHHs from a Naive Phage Display Selection.2,3
Fig. 1 Identification and Analysis of CHIKV E2-targeting VHHs from a Naive Phage Display Selection.2,3
This study identified 20 VHH antibodies against the CHIKV E2 glycoprotein from an alpaca naive phage display library. It also illustrated the expression and purification of the selected VHH antibodies and validated their specificity to the CHIKV E2 protein
and relevant binding kinetics, revealing that Nb-2E8 and Nb-3C5 exhibit high-affinity binding in the nanomolar range, with strength 100 times greater than that of monovalent antibodies. The aforementioned Fig.1 describes the screening process of VHH
antibodies from the phage display library and the identification of 20 unique antibodies. It demonstrates the effectiveness of the screening method and the diversity of the selected antibodies, providing candidate antibodies for further functional
studies. Overall, this research presents a universal method of CHIKV E2-targeting VHH discovery using a phage library and offers a novel strategy for the early diagnosis of CHIKV.
References
-
Ledsgaard, Line, et al. "Basics of antibody phage display technology." Toxins 10.6 (2018): 236.
-
Li, Qianlin, et al. "Highly potent multivalent VHH antibodies against Chikungunya isolated from an alpaca naïve phage display library." Journal of Nanobiotechnology 20.1 (2022): 231.
-
Distributed under Open Access license CC BY 4.0, without modification.
FAQ
1. Q: How to calculate the capacity of constructed library?
The capacity of constructed library can be evaluated by the number of colonies present on the transformed plates with serial repeated dilutions. Calculate the average of all dilution plates after counting colonies, which indicates the library size (cfu/mL).
2. Q: How to validate the quality of constructed library?
The quality of constructed library can be determined through QC colony PCR. Some colonies are randomly picked from the titration plates and used for colony PCR to analyze the insertion of the correct size.
3. Q: How to evaluate the diversity of constructed library?
The diversity of constructed library can be determined through QC sequencing. Some colonies are randomly picked from the titration plates and used for sequencing and alignment to evaluate the diversity. It is well known that most of the diversity of the
antibody molecule is found in the complementarity-determining regions (CDRs).
4. Q: How to choose between the immune library and premade library?
Immune library is the best-fit choice for specific project purposes, such as some specific target antigens, the high-affinity antibody requirement from nanomoles to picomoles, the largest possible number of antibody clones, improved probability to obtain
the exact immune response.
Creative Biolabs can provide several excellent premade libraries which have great diversity to be able to derive specific antibodies. They can be used for rapid discovery of high-potency sdAb against any targets and allow to save your
time and cost from library construction.
5. Q: Which kind of starting material can be accepted for naïve library construction?
In essence, the naïve library is constructed by the cDNA isolated from non-immune animals. The RNA from several animals can be mixed to generate one qualified naïve library.
Creative Biolabs can accept properly stored tissues and RNA as starting materials for library construction. Please note, at least 50 μg total RNA or 10 μg mRNA is required for one library construction project.
6. Q: How to identify specific binders after the screening process?
After the screening process, Creative Biolabs can offer two general strategies to identify target-specific binders and then perform corresponding functional assays to select desired sdAb candidates.
Strategy 1: Monoclonal Phage-based Binder Identification & Validation
To isolate specific binders against the target of interest, a group of monoclonal phage clones is randomly picked and gone through a series of validation
processes to obtain the sequence of identified positive clones. We can also generate the purified sdAbs for further characterization assays, such as affinity measurement.
Strategy 2: Magic™ Therapeutic Antibody Discovery Platform-based Binder Identification & Validation
Creative Biolabs has established the Magic™ Therapeutic Antibody Discovery Platform which is an exclusive high-throughput
solution for the identification of all potential novel binders at one-time. Purified sdAbs can be generated from the sequence of identified clones for further characterization assays, such as affinity measurement.
7. Q: What is the lead time for a general sdAb discovery project?
In general, we need around 6-9 months to complete an immunization-based novel sdAb discovery project, thereinto, the immunization process will spend about 3 months to achieve acceptable immune responses. By screening our premade libraries, only 3-4 months
is required to identify new sdAb binders.
8. Q: Are there any additional deliverables available for our internal test?
Yes. Generally, we can provide small aliquots of outputs (phages) after library screening as well as supernatants of validated positive clone (phage-free), which may vary due to different project design. Please note, these materials are not included in
the default deliverable list because the stability cannot be 100% guaranteed (especially for oversea delivery). Please notify us at the early stage if you want to receive these materials, they may be charged accordingly.
9. Q: Could the helper phage be titrated by blue-white screening?
The helper phage provided by Creative Biolabs does not contain lacZα but carries the kanamycin-resistant gene for antibiotic selection, thus cannot be distinguished through blue-white screening.
10. Q: What is the difference between pfu and cfu?
The pfu, refers to the plaque-forming unit, is a measure of the quantity of individual infectious particles, and is usually used to count bacteriophage.
The cfu, refers to the colony-forming unit, is a measure of viable cells in which a colony represents an aggregate of cells derived from a single progenitor cell, and is usually used to count bacteria (e.g. E. coli).
11. Q: Are anti-M13 antibodies available?
Yes. Recombinant anti-M13 Major Coat Protein Antibody (CBMAB-0206MC) or other custom anti-M13 antibodies are available of your choice. Please
inquire us for more details.
12. Q: Deliverables of Library Construction & Screening Stage
1. Project report including the library construction & screening processes.
2. Constructed library (do not apply to premade library screening).
3. Sequence information of the final identified positive binder(s).
Resources
We are offering highly customized CRO services to assist your Single Domain Antibody (sdAb) related projects. Please Contact Us for more details.


 Fig. 1 Identification and Analysis of CHIKV E2-targeting VHHs from a Naive Phage Display Selection.2,3
Fig. 1 Identification and Analysis of CHIKV E2-targeting VHHs from a Naive Phage Display Selection.2,3