Researchers from the U.S. Army Medical Research Institute of Infectious Diseases and Moderna published a research paper titled “Comparison of protection against mpox following mRNA or modified vaccinia Ankara vaccination in nonhuman primates” in the top international academic journal Cell.
The study compared the effectiveness of the MVA vaccine and the mRNA-1769 vaccine in non-human primates. The results showed that, similar to MVA, mRNA-1769 produced protection against monkeypox virus challenge and further reduced symptoms and disease course. Compared with MVA, mRNA-1769 enhanced viral control and disease reduction, highlighting the potential of mRNA vaccines to mitigate the threat of future pandemics.
Alec Freyn, a virology researcher at Moderna, said the study is the first to directly compare an investigational mRNA monkeypox vaccine with the current standard vaccine in a non-human primate model. When these vaccines are used directly in primates, we see positive effects of the mRNA vaccines, not only improving survival rates, but also reducing lesions and shortening the duration of the disease.
The MVA vaccine, originally developed to fight smallpox, contains an intact virus that has been weakened so it cannot cause disease in humans. However, this weakening also means that the MVA vaccine offers limited protection compared with other vaccines, such as the potent but potentially infectious ACAM2000. In contrast, using mRNA technology allows vaccines to include only the parts of the virus most likely to elicit a durable, protective immune response, without exposing people to the entire infectious virus. In this case, the monkeypox mRNA vaccine under study is composed of four viral antigens that are critical for the virus to attach to and enter host cells.
Galit Alter, corresponding author of the paper and virologist and immunologist at Moderna, said that with the mRNA vaccine, we can select the virus fragments that can produce the most effective immune response. This way, you can pinpoint your virus protection circle instead of being distracted by all the viruses.
Although there are studies showing that mRNA vaccines can prevent infection in non-human primates, their ability to limit disease severity has not been tested before. To directly compare the mRNA and MVA vaccines, the researchers vaccinated macaque monkeys and then exposed them to a deadly strain of monkeypox virus eight weeks after the initial vaccination. They also exposed six unvaccinated animals to the virus as a control group. After infection, the researchers monitored the animals’ health for four weeks and collected blood samples to check their immune responses.
Regardless of which vaccine was used, all 12 vaccinated animals survived, while five of the six unvaccinated animals succumbed to the disease. While both vaccines reduced disease severity compared with the control group, animals vaccinated with the mRNA vaccine lost less weight and had fewer lesions than animals vaccinated with the MVA vaccine—on average, the animals in the control group had a maximum of 1448 lesions, MVA-vaccinated animals had a maximum of 607 lesions, and mRNA-vaccinated animals had a maximum of 54 lesions. The mRNA vaccine also reduced disease duration (the number of days animals develop lesions) by more than 10 days compared with the MVA vaccine and resulted in lower viral loads in blood and throat swabs, suggesting that the mRNA vaccine may also have a role in reducing transmission. More effective.
Jay Hooper, corresponding author of the paper and a virologist at the U.S. Army Medical Research Institute of Infectious Diseases, said that with mRNA technology, we can produce a vaccine that can produce a fairly effective response and has a very good safety profile. We have been working hard to develop a vaccine that prevents the spread of the virus, like the ACAM2000 vaccine, but without the safety issues. Our research suggests that mRNA technology may fill this gap.
When the researchers compared the immune responses elicited by the mRNA vaccine and the MVA vaccine, they found that the mRNA vaccine produced higher numbers of antibodies with more diverse immune functions. The study also identified different types of antibodies that were associated with enhanced viral control and fewer lesions.
The mRNA vaccine has also shown the potential to induce cross-immunity to other poxviruses, whereas the MVA vaccine generates a smaller immune response and is less potent in neutralizing distantly related poxviruses.
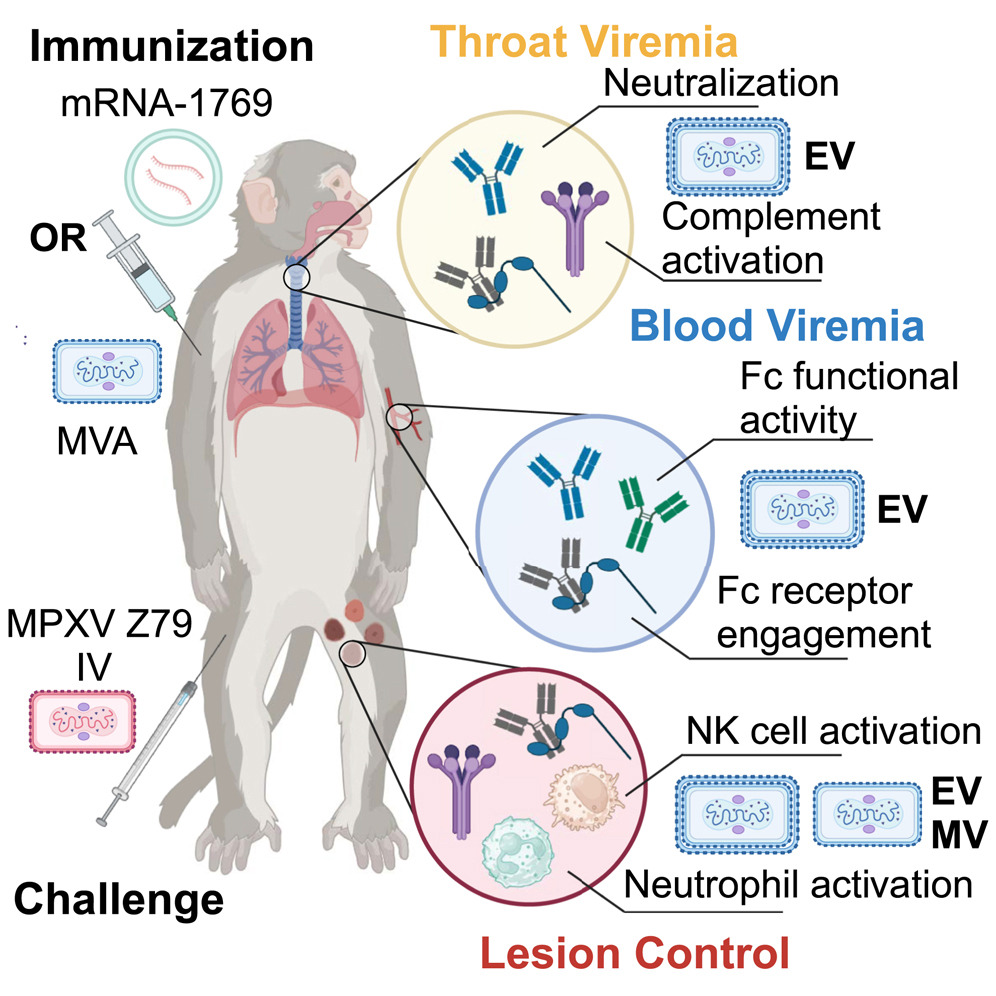
Fig. 1 The mechanism diagram of monkeypox vaccine in animal models.1
Alec Freyn said, “We tested the serum of monkeys that had been vaccinated with this vaccine, and these monkeys were basically resistant to all the poxviruses that we could come into contact with.” It neutralizes not only monkeypox, but also viruses such as cowpox, rabbitpox, camelpox and sheeppox. We believe this monkeypox virus vaccine will protect against other poxvirus threats that may arise in the future.
It is reported that Moderna’s mRNA-1769 vaccine is currently being evaluated in a Phase 1/2 clinical trial (NCT05995275) to determine the safety, tolerability and immune response of a range of doses of the mRNA-1769 vaccine in humans.
Conduct better research using our monkeypox virus products:
Antibodies
| CAT | Product Name |
| VASX-0522-SX4 | Anti-Monkeypox Virus Monoclonal Antibody (VACV-5C7), Mouse IgG1, Kappa |
| VASX-0522-SX5 | Anti-Monkeypox Virus Monoclonal Antibody (VACV-5C7), Human IgG1, Kappa |
| VASX-0522-SX6 | Anti-Monkeypox Virus Monoclonal Antibody (VACV-5C7), Human IgG1, Fc Silent, Kappa |
| VASX-0522-SX7 | Anti-Monkeypox Virus Monoclonal Antibody (VACV-5C7), Human IgM, Kappa |
| VASX-0522-SX8 | Anti-Monkeypox Virus Monoclonal Antibody (VACV-5C7), Rabbit IgG, Kappa |
| VASX-0522-SX9 | Anti-Monkeypox Virus & Vaccinia A13 Monoclonal Antibody (11F7), Rabbit IgG, Kappa |
Recombinant Protein
| CAT | Product Name |
| VASX-0622-SX1 | Recombinant Monkeypox L1R Protein (aa 1-152) [His] |
| VASX-0622-SX2 | Recombinant Monkeypox A29 Protein (aa 21-110) [His] |
Detection Kits
| CAT | Product Name |
| VASX-0522-SX1 | Monkeypox Virus (MPV) Real Time PCR Kit |
| VASX-0522-SX2 | Monkeypox Virus (MPV) Antigen ELISA Kit |
| VASX-0522-SX3 | Monkeypox Virus (MPV) IgG ELISA Kit |
Reference
- Mucker, Eric M., et al., “Comparison of protection against mpox following mRNA or modified vaccinia Ankara vaccination in nonhuman primates.” Cell(2024).
