Antibodies are proteins that can bind to specific molecules called antigens, and are widely used for therapeutic purposes. However, conventional antibodies, also known as monoclonal antibodies (mAbs), can only bind to one antigen at a time, limiting their effectiveness and versatility. To overcome this limitation, researchers have developed bispecific antibodies (bsAbs), which are antibodies that can bind to two different antigens simultaneously. BsAbs have several advantages over mAbs, such as increased potency, specificity, functionality, and diversity. BsAbs can be used to target multiple pathways or mechanisms involved in various diseases, especially cancer. However, bsAbs also pose some challenges, such as complex design, engineering, production, and characterization. Therefore, different formats and technologies have been developed to generate bsAbs with different structures and properties. One of these formats is the biparatopic bsAb, which is a bsAb that targets two distinct epitopes on the same antigen. Biparatopic bsAbs have shown promising results in preclinical and clinical studies, demonstrating enhanced binding affinity, avidity, specificity, and functionality.
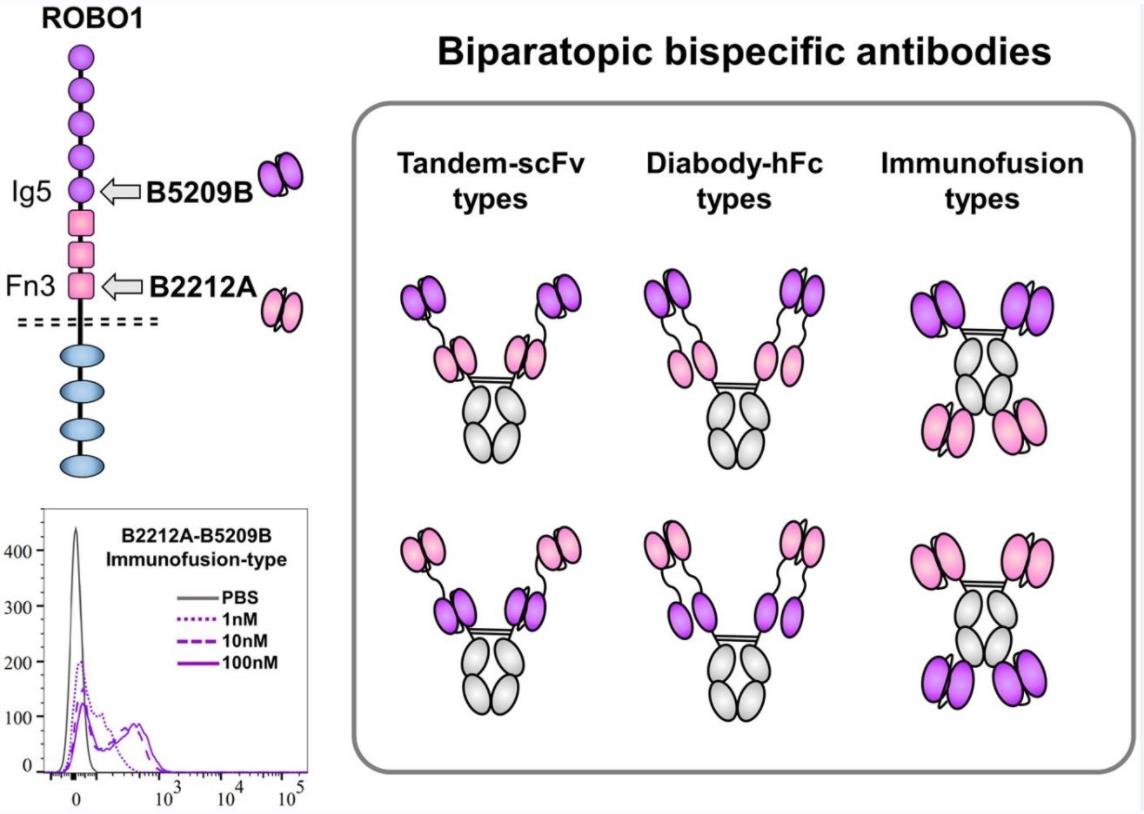
Fig.1 Biparatopic bsAb (Watanabe, 2021)
Principle of Biparatopic BsAbs
Biparatopic bsAbs are bsAbs that target two distinct epitopes on the same antigen. Epitopes are the specific regions on the antigen that are recognized and bound by the antibody. By targeting two epitopes, biparatopic bsAbs can achieve higher binding affinity and avidity than monospecific or monovalent antibodies, which target only one epitope. Affinity is the strength of the interaction between one antibody and one antigen, while avidity is the overall strength of the interaction between multiple antibodies and multiple antigens. Biparatopic bsAbs can also improve the specificity and selectivity of the antibody by reducing off-target binding and minimizing side effects. Furthermore, biparatopic bsAbs can overcome or delay the development of resistance by targeting multiple epitopes or pathways that are essential for the survival or growth of the target cells.
Biparatopic bsAbs can be generated using different technologies, such as chemical conjugation, hybridoma fusion, genetic engineering, or phage display. Chemical conjugation involves linking two different antibodies or antibody fragments with a chemical linker, such as a thioether or a disulfide bond. Hybridoma fusion involves fusing two different hybridoma cells, which are cells that produce antibodies, to create a quadroma cell that produces a bsAb. Genetic engineering involves manipulating the DNA sequences of antibody genes to create a single gene that encodes a bsAb. Phage display involves displaying antibody fragments on the surface of bacteriophages, which are viruses that infect bacteria, and selecting the ones that bind to the target antigen.
Different formats and structures have been developed to address these challenges and create diverse and complex biparatopic bsAbs. Some examples of these formats are IgG-scFv (immunoglobulin G-single-chain variable fragment), dual variable domain-immunoglobulin, bispecific T-cell engager, dual-affinity re-targeting, and Fab-arm exchange (FAE). These formats differ in their valency, size, flexibility, and stability, as well as their production methods and clinical applications.
How Biparatopic BsAbs Outperform Other Antibodies
Biparatopic bsAbs have several features that make them superior to other types of bsAbs or mAbs. First, they have higher binding affinity and avidity, which means they can bind more strongly and stably to the target antigen, increasing the potency and efficacy of the antibody. This is because they can crosslink and aggregate the target antigen, creating a stronger interaction than monospecific or monovalent antibodies that target only one epitope. Second, they have improved specificity and selectivity, which means they can bind more accurately and exclusively to the target antigen and cells, reducing off-target effects and side effects. This is because they can avoid binding to other antigens that share one epitope with the target antigen, and discriminate between normal and malignant cells. Third, they have enhanced functionality, which means they can perform more actions and functions than monospecific or monovalent antibodies, such as blocking, signaling, recruiting, or activating other molecules or cells. This is because they can target multiple epitopes or pathways that are involved in various biological processes or mechanisms. Fourth, they have reduced resistance, which means they can maintain their effectiveness and durability over time, preventing or delaying the escape or mutation of the target antigen or cells. This is because they can target multiple epitopes or pathways that are essential for the survival or growth of the target cells. These features make biparatopic bsAbs attractive candidates for various therapeutic applications, especially in oncology.
The Potential of Biparatopic BsAbs in Various Fields
Biparatopic bsAbs have diverse and versatile applications in various fields, especially in oncology. In oncology, biparatopic bsAbs can target various tumor-associated antigens that are involved in the growth, survival, or metastasis of cancer cells. Besides oncology, biparatopic bsAbs also have potential applications in other fields, such as infectious diseases, autoimmune diseases, or neurological diseases.
Table 1. Examples of biparatopic bsAbs and their applications
|
Target antigen
|
Epitope 1
|
Epitope 2
|
Disease
|
Anti-tumor effect
|
|
HER2
|
Domain I
|
Domain IV
|
Breast cancer
|
Blocking signaling pathways, enhancing ADCC
|
|
EGFR
|
Domain III
|
Domain IV
|
Colorectal cancer
|
Blocking signaling pathways, overcoming resistance to tyrosine kinase inhibitors
|
|
CD20
|
Loop 1
|
Loop 2
|
Lymphoma
|
Recruiting immune cells, activating complement system, enhancing ADCC
|
|
Anthrax toxin
|
Protective antigen domain 4
|
Lethal factor domain 1
|
Anthrax infection
|
Neutralizing and clearing the toxin
|
|
TNF-alpha
|
Site 1
|
Site 2
|
Rheumatoid arthritis
|
Modulating immune response, reducing inflammation and tissue damage
|
|
Amyloid-beta
|
N-terminal region
|
Central region
|
Alzheimer's disease
|
Solubilizing and clearing the protein aggregates, reducing neuronal dysfunction and death
|
These are some examples of biparatopic bsAbs that target two distinct epitopes on the same antigen and have various applications in different fields. Like the aforementioned, biparatopic bsAbs can achieve higher binding affinity, avidity, specificity, selectivity, functionality, and resistance than monospecific or monovalent antibodies. They can also induce different effects depending on the target antigen and the disease. Biparatopic bsAbs are promising candidates for therapeutic biologics that can offer tailor-made functional properties and overcome the limitations of conventional antibodies.
References
1. Li J, et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer Cell. 2016 Jan 11;29(1):117-129.
2. Li L, et al. A novel biparatopic EGFR-specific antibody with potent antitumor activity in vitro and in vivo. Oncotarget. 2017 May 23;8(21):34671-34684.
3. Watanabe Y, et al. Development of biparatopic bispecific antibody possessing tetravalent scFv-Fc capable of binding to ROBO1 expressed in hepatocellular carcinoma cells. J Biochem. 2021 Oct 11;170(2):307-315.
4. Li F, et al. A novel CD20/CD3 bispecific antibody exhibits potent cytotoxicity for human B-cell lymphoma. Int J Cancer. 2018 Feb 15;142(4):815-825.
5. Zhang W, et al. A novel biparatopic anthrax toxin-neutralizing antibody that confers protection in two in vivo anthrax infection models. MAbs. 2015;7(5):960-971.
6. Liu X, et al. A bispecific antibody targeting amyloid-beta and transthyretin prevents amyloid-beta toxicity and aggregation in vitro and in vivo. Transl Neurodegener. 2019 Dec 16;8:35.
7. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
8. Kontermann RE, et al. Bispecific antibodies: a historical perspective and guide to the future. Nat Rev Drug Discov. 2020 Jul;19(7):477-494.
9. Brinkmann U, et al. Bispecific antibodies for cancer immunotherapy: current perspectives. BioDrugs. 2018 Aug;32(4):347-362.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










