Monoclonal antibodies (mAbs) are a class of biologic drugs that are produced by cloning a single B cell that recognizes a specific antigen. They have been widely used for the diagnosis, prevention, and treatment of various diseases, especially cancer, since the first mAb was approved by the FDA in 1986. mAbs can bind to specific antigens on the surface or inside of target cells or molecules and trigger different immune responses that can eliminate or inhibit the growth of pathogens or tumors. However, mAbs also face some challenges and limitations, such as immunogenicity, toxicity, and resistance, which can reduce their efficacy and safety. Therefore, understanding the inherent mechanisms of mAbs and developing strategies to overcome their drawbacks is crucial for improving their clinical outcomes and expanding their applications.
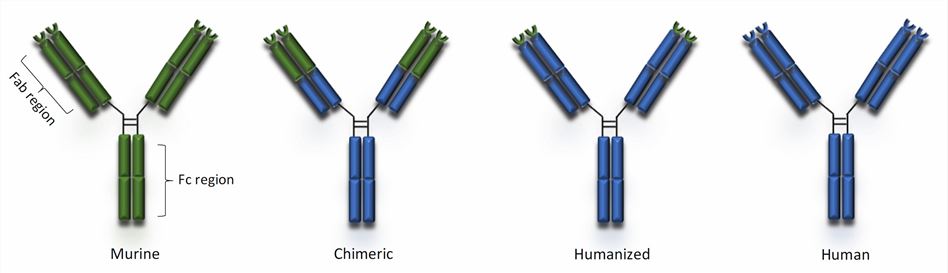
Fig.1 Types of monoclonal antibodies (Gklinos, 2021)
Mechanisms of Action of Monoclonal Antibodies
mAbs exert their therapeutic effects by binding to specific antigens on target cells or molecules and triggering different immune responses that can eliminate or inhibit the growth of pathogens or tumors. The binding affinity and specificity of mAbs depend on several factors, such as antigen density, epitope accessibility, and antibody isotype. The immune responses elicited by mAbs can be classified into two main categories: effector-dependent and effector-independent.
Effector-dependent mechanisms involve the recruitment and activation of immune cells or molecules that mediate the killing or neutralization of the target. These include antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and phagocytosis. ADCC occurs when mAbs bind to Fc receptors on natural killer (NK) cells, macrophages, or neutrophils and induce them to release cytotoxic granules or cytokines that destroy the target cell. CDC occurs when mAbs activate the complement system, which forms a membrane attack complex (MAC) that lyses the target cell. Phagocytosis occurs when mAbs opsonize the target cell, which facilitates its ingestion and degradation by macrophages or neutrophils.
Effector-independent mechanisms involve the direct modulation of signaling pathways or functions of the target cell or molecule. These include apoptosis, receptor blockade, receptor downregulation, and signal transduction inhibition. Apoptosis occurs when mAbs induce intrinsic or extrinsic pathways of programmed cell death in the target cell. Receptor blockade occurs when mAbs prevent the binding of ligands to receptors on the target cell, inhibiting its activation or proliferation. Receptor downregulation occurs when mAbs induce the internalization and degradation of receptors on the target cell, reducing its sensitivity or responsiveness. Signal transduction inhibition occurs when mAbs interfere with downstream signaling molecules or pathways that mediate the survival or growth of the target cell.
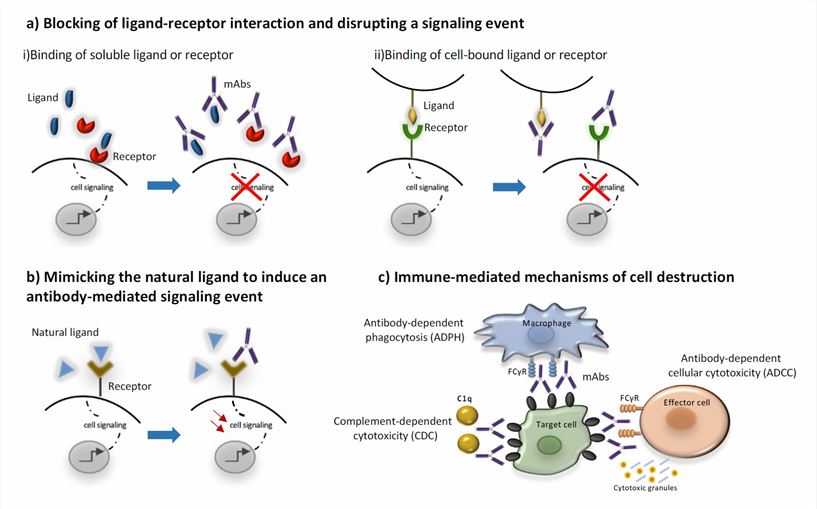
Fig.2 Mechanisms of action of monoclonal antibodies (Gklinos, 2021)
Mechanisms of Resistance to Monoclonal Antibodies
mAbs can encounter various inherent mechanisms that confer resistance to their therapeutic effects, either before or after they bind to their target antigens. These mechanisms can be classified into two main categories: antigen-related and effector-related.
Antigen-related mechanisms involve the alteration or loss of the target antigen, reducing or abolishing the binding of mAbs. These include antigen mutation, antigen masking, antigen shedding, and antigen internalization. Antigen mutation occurs when the target antigen undergoes genetic or epigenetic changes affecting its expression or structure. Antigen masking occurs when the target antigen is hidden or blocked by other molecules, such as soluble ligands, extracellular matrix proteins, or glycans. Antigen shedding occurs when the target antigen is cleaved or released from the cell surface into the circulation, decreasing its density or availability. Antigen internalization occurs when the target antigen is endocytosed or recycled by the cell, reducing its exposure or accessibility.
Effector-related mechanisms involve the impairment or evasion of the immune responses triggered by mAbs. These include Fc receptor polymorphism, Fc receptor expression, complement inhibition, complement depletion, and immune suppression. Fc receptor polymorphism occurs when the Fc receptors on immune cells have different genetic variants affecting their affinity or function. Fc receptor expression occurs when immune cells modulate their Fc receptor levels or distribution in response to mAbs or other stimuli. Complement inhibition occurs when the target cells express or secrete molecules that interfere with complement activation or cascade. Complement depletion occurs when complement components are consumed or exhausted by repeated mAb administration or chronic inflammation. Immune suppression occurs when the target cells induce or recruit regulatory cells or factors that dampen or counteract immune activation or cytotoxicity.
Conclusion
In conclusion, mAbs are a powerful and versatile class of biologic drugs that have revolutionized the diagnosis, prevention, and treatment of various diseases, especially cancer. By binding to specific antigens on target cells or molecules, mAbs can trigger different immune responses that can eliminate or inhibit the growth of pathogens or tumors. However, mAbs also face some challenges and limitations, such as immunogenicity, toxicity, and resistance, which can compromise their efficacy and safety. Therefore, it is essential to understand the inherent mechanisms of mAbs and develop strategies to overcome their drawbacks. Some of the potential strategies include optimizing mAb design and engineering, combining mAbs with other agents or modalities, targeting multiple antigens or pathways, personalizing mAb therapy based on biomarkers or pharmacokinetics, and enhancing immune surveillance and activation. These strategies can improve the clinical outcomes and expand the applications of mAbs. However, there are still some gaps and challenges in mAb research and development. These include identifying novel antigens or biomarkers, understanding the complex interactions between mAbs and the immune system or the tumor microenvironment, designing more effective and safer mAb formats or combinations, and addressing the ethical, regulatory, and economic issues of mAb therapy. Therefore, more studies are needed to address these issues and advance the field of mAb therapeutics.
References
1. Lu RM, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci. 2020 Jan 2;27(1):1. 1
2. Beck A, et al. The next generation of antibody drug conjugates comes of age. Discov Med. 2010 Oct;10(53):329-39.
3. Weiner LM, et al. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010 May;10(5):317-27.
4. Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015 Jun;15(6):361-70.
5. Scott AM, et al. Antibody therapy of cancer. Nat Rev Clin Oncol. 2012 Mar 6;9(4):199-212.
6. Carter PJ, et al. Next generation antibody drugs: pursuit of the "high-hanging fruit". Nat Rev Drug Discov. 2018 Jan;17(3):197-223.
7. Nelson AL, et al. Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov. 2010 Oct;9(10):767-74.
8. Reichert JM, et al. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005 Sep;23(9):1073-8.
9. Holliger P, et al. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005 Sep;23(9):1126-36.
10. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
11. Weiner LM, et al. Antibody-based immunotherapy of cancer. Cell. 2012 Apr 13;148(6):1081-4.
12. Gklinos P, et al. Monoclonal Antibodies as Neurological Therapeutics. Pharmaceuticals (Basel). 2021 Jan 26;14(2):92.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY











