What is IgG(H)-scFv?
IgG(H)-scFv is a fusion protein composed of immunoglobulin G (IgG) and a single-chain variable fragment (scFv). The scFv is connected to or inserted into different positions of a complete IgG molecule, providing dual-site binding and dual antibody functions. In IgG(H)-scFv, the scFv is typically located at the C-terminal of IgG and connected to the C-terminal of IgG, forming a complete fusion protein. The molecular structure of this fusion protein enables it to possess both the Fc region function of IgG and the antigen specificity of scFv.
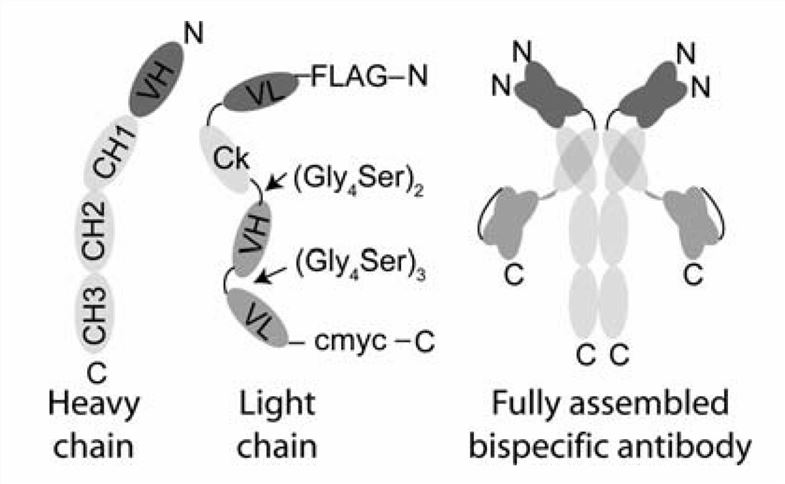
Fig.1 Design of IgG-scFv (Orcutt KD, 2010)
Generation methods of IgG(H)-scFv
The common methods for generating IgG-scFv are as follows:
1. Quadroma Approach: This method involves the fusion of two different hybridoma cell lines, each producing a monoclonal antibody with specific binding properties. By fusing these hybridoma cells, a quadroma cell line is generated, which can produce bispecific IgG-scFv antibodies.
2. Genetic Engineering: Recombinant DNA technology can be used to engineer IgG-scFv antibodies. This involves cloning and expression of separate genes encoding the heavy chain of IgG and the scFv region. These genes are then expressed in appropriate host cells to produce the desired IgG-scFv fusion protein.
3. Antibody Fragment Fusion: In this method, the scFv region is fused directly to the C-terminus of the heavy chain of IgG. This fusion can be achieved through genetic engineering techniques or recombinant protein expression.
4. Protein Engineering: Various protein engineering techniques, such as site-directed mutagenesis or domain swapping, can be employed to create IgG-scFv antibodies. These methods involve modifying the structure of IgG antibodies to incorporate the scFv region while maintaining their binding properties.
Advantages and disadvantages of IgG(H)-scFv
Fusion of IgG(H)-scFv allows interaction with Fc receptors present in the immune system, thereby activating cellular cytotoxicity, modulating immune responses, or mediating phagocytosis, among other effects. Additionally, due to the highly specific binding activity of the scFv region towards a particular antigen, this fusion protein can also serve as an antigen recognition molecule for targeting specific antigens in therapeutic, diagnostic, or research applications. In addition, monoclonal antibodies (mAbs) in the form of scFv entities are superior to conventional antibodies as they have smaller sizes, thus reducing the likelihood of generating an anti-mouse antibody response. Development of smaller antibody fragments such as Fab and scFv, compared to IgG 11-25 mAb, will contribute to faster blood clearance and improved "target-to-non-target" contrast at early time points after administration. In addition to their role as therapeutic agents, another important application of scFv is in diagnostic applications, where anti-MSLN scFv can clearly visualize MSLN-expressing tumors through PET imaging within 3 hours post-injection.
Clinical data of IgG(H)-scFv
Multiple IgG-scFv bispecific antibodies (BsAbs) have been developed for the specific purpose of Pretargeted Radioimmunotherapy (PRIT). These BsAbs are composed of an IgG chain, which specifically targets cancer cell surface antigens such as carcinoembryonic antigen (CEA) or A33, connected with a C825 scFv to form an IgG-scFv format. These IgG-scFv BsAbs have demonstrated sufficient functionality and stability in vivo, enabling efficient targeting of radiolabeled agents like 177Lu-DOTA in cancer cells.
The IgG-scFv bispecific dual antagonist antibody is designed to simultaneously target human B cell activating factor (BAFF) and interleukin-17A (IL-17). The anti-IL-17 single-chain variable fragment (scFv) derived from ixekizumab (Taltz®) is fused to the anti-BAFF monoclonal antibody with a glycine-rich linker. The stable bispecific molecule LY3090106 (tibulizumab) effectively antagonizes BAFF and IL-17 in cellular and murine models. In cynomolgus monkeys, it inhibits B cell development and survival while maintaining functional integrity and an extended half-life in circulation. It holds great potential for clinical development.
References
1. ORCUTT K D, ACKERMAN M E, et al."A modular IgG-scFv bispecific antibody topology." Protein engineering, design & selection : PEDS, 2010, 23(4): 221-8.
2. KLEIN C, SUSTMANN C, et al."Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies." mAbs, 2012, 4(6): 653-63.
3. CAO M, WANG C, et al."Characterization and analysis of scFv-IgG bispecific antibody size variants." mAbs, 2018, 10(8): 1236-47.
4. CHEAL S M, XU H, et al."Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex." Molecular cancer therapeutics, 2014, 13(7): 1803-12.
5. LIM M S H, OHTSUKI T, et al."A Novel (89)Zr-labeled DDS Device Utilizing Human IgG Variant (scFv): "Lactosome" Nanoparticle-Based Theranostics for PET Imaging and Targeted Therapy." Life (Basel, Switzerland), 2021, 11(2).
6. SCHNEIDER K T, KIRMANN T, et al."Shelf-Life Extension of Fc-Fused Single Chain Fragment Variable Antibodies by Lyophilization." Frontiers in cellular and infection microbiology, 2021, 11: 717689.
7. DIMASI N, FLEMING R, et al."Molecular engineering strategies and methods for the expression and purification of IgG1-based bispecific bivalent antibodies." Methods (San Diego, Calif), 2019, 154: 77-86.
8. BENSCHOP R J, CHOW C K, et al."Development of tibulizumab, a tetravalent bispecific antibody targeting BAFF and IL-17A for the treatment of autoimmune disease." mAbs, 2019, 11(6): 1175-90.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










