Creative Biolabs is a leading service provider that focuses on polyclonal and monoclonal antibody development for research, diagnosis, and potential therapeutics. Based on our extensive experience and state-of-the-art platforms, Creative Biolabs now offers a series of biomarker-specific IVD (in vitro diagnostic) antibody development services to clients globally. We are confident that our commitment to science and research will enable us to offer you the best products and services. Here, we introduce our IVD antibody development services for the Epstein-Barr virus (EBV).
Introduction of EBV
The Epstein–Barr virus (EBV), also called human herpesvirus 4 (HHV-4), is one of eight known human herpesvirus types in the herpes family that has a double-stranded DNA genome of 184-kb pairs in length, encoding nearly 100 proteins. As one of the most common viruses in humans, EBV is commonly transmitted by oral contact with saliva such as exchange of saliva among young children directly or through the handling of toys or by kissing among adolescents. Epstein-Barr virus infection results in a spectrum of diseases, with the host immune response playing a key role in shaping the clinical manifestations. In addition, infectious mononucleosis the prototype EBV infection and is characterized by fever, sore throat, cervical and generalized lymphadenopathy, hepatosplenomegaly, and somatic complaints of fatigue and malaise.
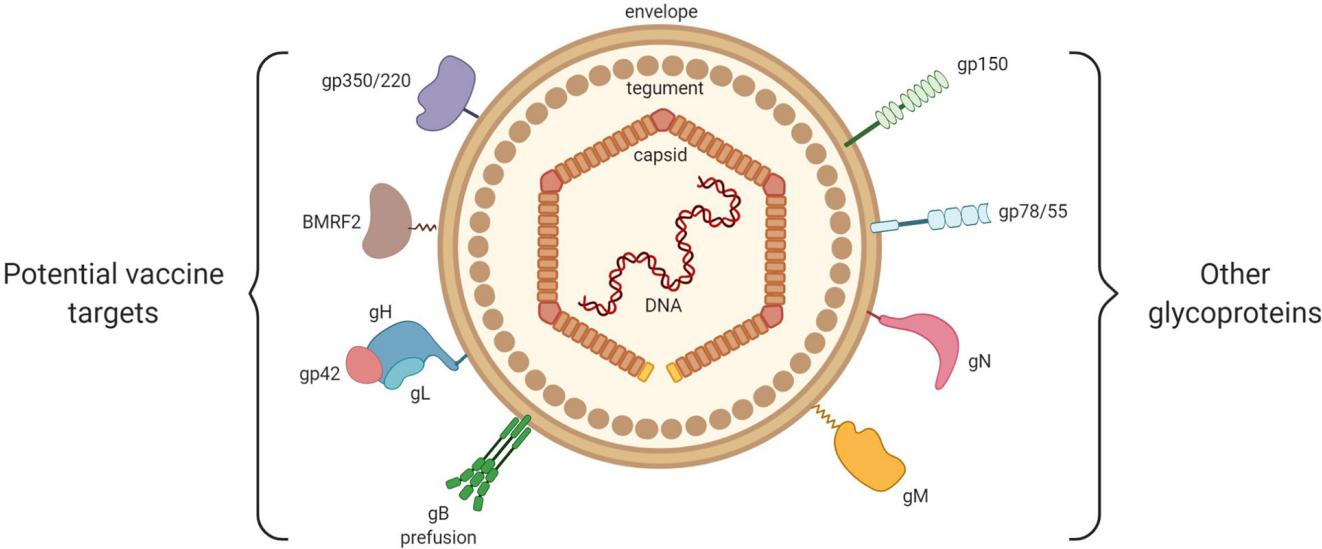 Fig.1 Epstein–Barr virus structure.1
Fig.1 Epstein–Barr virus structure.1
Correlation of EBV and Childhood
It has been reported that infections of EBV are associated with age. In developing countries and among socioeconomically disadvantaged populations of developed countries, transmission of EBV occurs almost universally during infancy and early childhood. In central Africa, almost all children are infected with EBV by 3 years of age, resulting in asymptomatic infection or mild disease that is indistinguishable from many other childhood infections. Most importantly, primary infection with EBV in early childhood is often asymptomatic or subclinical but results in a lifelong dormant infection. What’s more, when primary EBV infection occurs during adolescence or later in life, it manifests as the clinical syndrome of infectious mononucleosis.
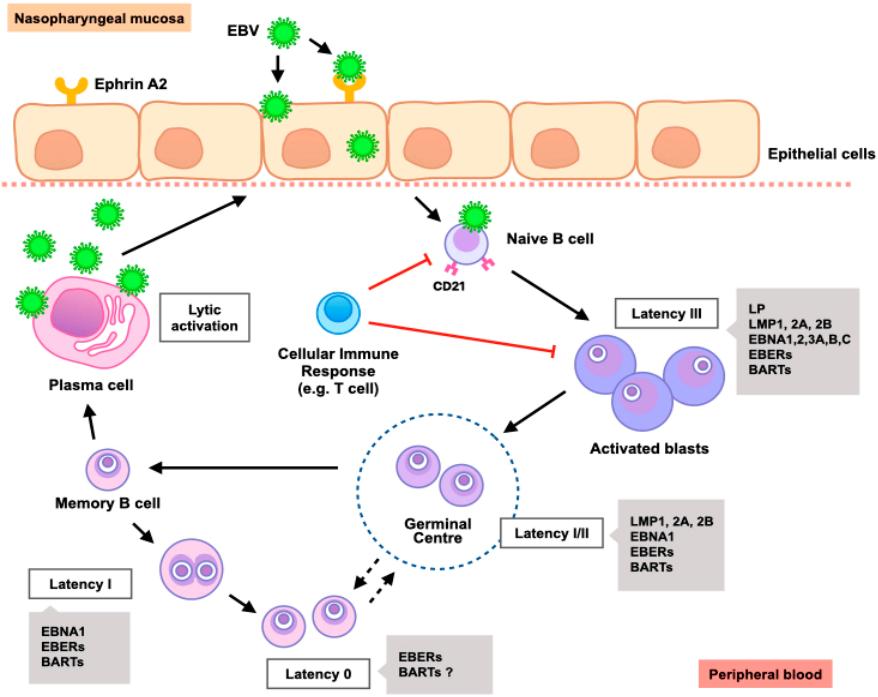 Fig.2 EBV life cycle.2
Fig.2 EBV life cycle.2
IVD Antibody Development Services for EBV
In the past years, with the rapid development of IVD technology, IVD antibodies have been widely used as important tools for the screening and diagnosis of numerous diseases. As a professional service provider of antibody development, Creative Biolabs provides customized IVD antibody development services for EBV. Besides antibody generation, Creative Biolabs also offers diagnostic immunoassay development services, including feasibility analysis, assay design, assay protocol establishment, assay optimization, and kit production.
If you are interested in the service we provide, please don’t hesitate to contact us or directly send us an inquiry. We will get in touch with you as soon as possible.
References
- Jean-Pierre, Vincent, et al. "Main targets of interest for the development of a prophylactic or therapeutic Epstein-Barr virus vaccine." Frontiers in Microbiology 12 (2021): 701611. Distributed under Open Access license CC BY 4.0, without modification.
- Richardo, Timmy, et al. "Epstein-Barr virus mediated signaling in nasopharyngeal carcinoma carcinogenesis." Cancers 12.9 (2020): 2441. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.

