Creative Biolabs stands as a world-class leader in the in vitro diagnostics (IVD) market, leveraging years of proven expertise in IVD antibody and kit development, particularly in advanced solutions like latex particle-enhanced turbidimetric immunoassay (LETIA) based kits. Our professional scientists and experienced staff operate cutting-edge experimental processes and machinery, ensuring all client project requirements are met with precision.
Latex Particle-Enhanced Turbidimetric Immunoassay
LETIA represents a significant advancement in quantitative diagnostic methods. This technique utilizes microscopic latex particles, meticulously coated with specific antibodies or antigens, to enhance the detection of target analytes in biological samples. When the analyte binds to the coated particles, it induces agglutination, leading to the formation of larger immune complexes. The resulting increase in turbidity of the solution is then precisely measured by spectrophotometry. This amplification mechanism dramatically improves the assay's sensitivity and allows for accurate quantification of even low-concentration biomarkers, making it an indispensable tool in modern clinical diagnostics and research.
Advantages of Our LETIA Platform

LETIA offers distinct advantages that make it a preferred choice for high-performance diagnostic applications:
-
Enhanced Sensitivity: The particle-enhanced amplification significantly lowers the detection limit, enabling the accurate quantification of analytes present in minute quantities.
-
High Automation Compatibility: LETIA assays are inherently designed for seamless integration with automated clinical chemistry analyzers, facilitating high-throughput processing and reducing manual intervention.
-
Rapid Turnaround Time: Compared to multi-step immunoassays like ELISA, LETIA provides significantly faster results, crucial for timely clinical decisions and high-volume testing.
-
Superior Stability: Kits developed with LETIA technology often exhibit excellent calibration stability and consistent signal changes, ensuring assay robustness and reproducibility over extended periods.
-
Cost-Effectiveness: The efficiency gained through automation and optimized reagent consumption contributes to a more economical diagnostic solution without compromising performance.
-
Minimal Sample Requirements: The high sensitivity allows for accurate analysis using small sample volumes, preserving precious patient samples.
Our LETIA Based Kits Development Services
Creative Biolabs offers comprehensive custom LETIA-based kit development services, tailored to your specific diagnostic needs. Our expertise spans the entire development lifecycle, from initial concept to a fully validated, market-ready product. We focus on developing highly specific and sensitive assays for a wide range of biomarkers, ensuring optimal performance on automated platforms. Our service includes meticulous optimization of particle size, surface chemistry, and conjugation protocols to achieve superior signal-to-noise ratios and broad dynamic ranges, crucial for accurate quantitative analysis. We are committed to delivering robust and reliable diagnostic solutions that meet stringent regulatory and performance standards.
 Fig.1 Schematic illustrating one possible mechanism of how an antibody interferes with particle-enhanced turbidimetric immunoassays.1,4
Fig.1 Schematic illustrating one possible mechanism of how an antibody interferes with particle-enhanced turbidimetric immunoassays.1,4
Service Workflow of LETIA Based Kits Development
Our LETIA-based kit development projects follow a structured, step-by-step workflow to ensure successful and efficient outcomes:
01Initial Consultation & Feasibility
We begin with an in-depth discussion to understand your target analyte, desired assay performance, and specific application. Our team assesses the feasibility and outlines a preliminary project plan.
02Antigen/Antibody Sourcing & Characterization
High-quality, specific antibodies or antigens are selected and rigorously characterized for their binding affinity and specificity to the target analyte.
03Latex Particle Selection & Functionalization
We choose optimal latex particle sizes and surface chemistries (e.g., carboxylated, amino-functionalized) and functionalize them to enable stable covalent coupling of the biorecognition molecules.
04Conjugation Optimization
Proprietary conjugation protocols are optimized to achieve high-density, stable, and correctly oriented immobilization of antibodies/antigens onto the latex particles, minimizing non-specific binding.
05Assay Development & Optimization
This critical phase involves developing the core assay protocol, optimizing reagent concentrations, reaction kinetics, buffer systems, and instrument parameters to achieve target sensitivity, linearity, and dynamic range.
06Analytical Validation
Rigorous analytical validation is performed, including assessment of precision (intra- and inter-assay), accuracy, linearity, detection limits (LOD/LOQ), specificity, and interference studies.
07Documentation & Final Delivery
Comprehensive documentation, including detailed protocols, validation reports, and quality control specifications, is provided, culminating in the delivery of a high-quality, ready-to-manufacture LETIA-based kit.
Applications
Clinical Diagnostics
LETIA is extensively used for the quantitative measurement of various biomarkers in biological fluids, enabling:
-
Protein Quantification: Accurate determination of serum proteins, including acute-phase reactants (e.g., CRP), immunoglobulins, and specific disease markers.
-
Infectious Disease Markers: Rapid detection and quantification of antigens or antibodies for the diagnosis and monitoring of viral, bacterial, and fungal infections, such as SARS-CoV-2.
-
Hormone and Therapeutic Drug Monitoring: Precise measurement of hormone levels and therapeutic drug concentrations to guide patient treatment and ensure optimal dosing.
-
Oncology & Autoimmune Biomarkers: Detection of specific tumor markers (e.g., GP73) and autoimmune disease indicators for early diagnosis, prognosis, and treatment monitoring.
Environmental Monitoring
LETIA's high sensitivity allows for detecting trace contaminants like heavy metals, pesticides, or microbial agents in water. Its rapid results facilitate quick intervention, supporting public health and safety initiatives by ensuring compliance with environmental regulations.
Food Safety
LETIA assays provide rapid, reliable screening for allergens (e.g., peanuts, gluten), toxins (e.g., E. coli, Salmonella), and microbial contaminants in food products. This ensures compliance with safety regulations, prevents foodborne illnesses, and maintains consumer confidence through high-throughput testing.
Pharmaceutical & Biotechnology Research
Essential for drug discovery, development, and quality control, LETIA enables precise quantification of targets in complex matrices. It supports high-throughput screening of drug candidates, monitors drug concentrations in preclinical studies, and assesses impurities in biopharmaceutical products, offering a valuable tool across the drug development pipeline.
Published Data
1. Development of Three Monoclonal Antibodies Based Automatic LETIA
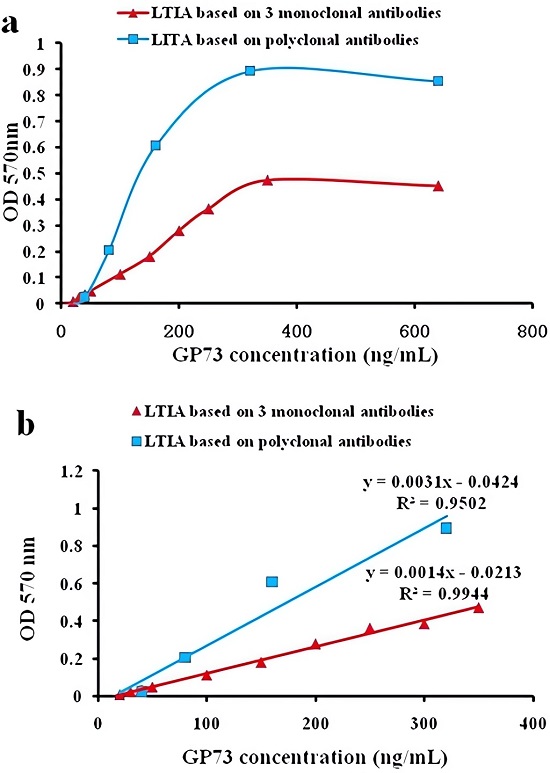 Fig.2 Linearity of LETIA/LTIA.2,4
Fig.2 Linearity of LETIA/LTIA.2,4
The recently introduced LETIA method, licensed for use in automated clinical chemistry analyzers, has not been extensively evaluated for its analytical performance using three monoclonal antibodies for Golgi protein 73 (GP73) measurement. Researchers developed a LETIA using latex beads immobilized with three GP73 monoclonal antibodies, optimizing experimental conditions and comparing its diagnostic potential, clinical relevance, and linearity to a polyclonal antibody-based LETIA and ELISA. The LETIA with monoclonal antibodies produced a calibration curve ranging from 10 to 350 ng/mL, while the polyclonal version ranged from 20 to 320 ng/mL. The detection limit was 1.82 ng/mL, with within-run CV between 1.5-2.9%. ROC analysis showed that the monoclonal antibody-based LETIA had sensitivity and specificity of 96.7% and 93.3%, outperforming the polyclonal LETIA (94.6% and 72.4%) and ELISA (70.0% and 83.3%), making it suitable for GP73 quantification in automated analyzers.
2. Bioinformatical Design and Performance Evaluation of Particle Enhanced Turbidimetric Immunoassay Based on a Nucleocapsid- and an RBD
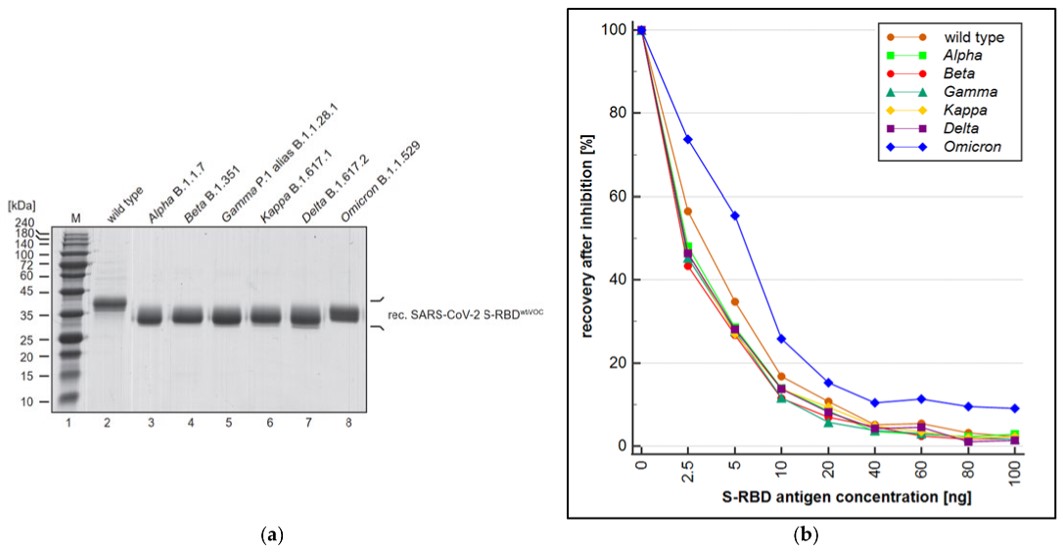 Fig.3 Evaluation of variant cross-reactivity via S-RBD-based PETIA.3,4
Fig.3 Evaluation of variant cross-reactivity via S-RBD-based PETIA.3,4
Researchers developed a bioinformatics-based approach to design two novel serological particle enhanced turbidimetric immunoassays (PETIA) for quantifying the SARS-CoV-2 immune response. They produced an S-RBD-based and a nucleocapsid-based PETIA and compared their sensitivity and specificity using 95 patient samples on an automated analyzer. The S-RBD-based PETIA demonstrated superior specificity over the N protein-based assay. Additionally, the reactivity and cross-reactivity of the RBD-based PETIA against variant-derived antibodies were assessed using a quenching inhibition test. This study highlights it is possible to rapidly design and develop specific and robust PETIA, which is crucial to address the continuous diagnostic challenges posed by the evolving variants of concern (VOC) during the progression of the pandemic.
Service Highlights
-
Tailored Solutions: We provide fully customized development, ensuring the final kit precisely meets your unique analyte, sensitivity, and application requirements, rather than offering generic solutions.
-
Advanced Particle Engineering: Leveraging expertise in polymer chemistry, we optimize latex particle properties (size, surface charge, functional groups) for superior conjugation efficiency and assay performance, directly impacting signal amplification.
-
Robust Conjugation Chemistry: Our specialized conjugation techniques ensure stable, high-density, and correctly oriented immobilization of biorecognition molecules, which is critical for assay specificity and long-term kit stability.
-
Comprehensive Validation: Beyond standard checks, we perform in-depth analytical and clinical validation studies, including interference testing and matrix effects, to guarantee the kit's reliability and accuracy in real-world samples.
-
Automation-Ready Design: From the outset, kits are designed for seamless integration with automated clinical analyzers, optimizing workflow efficiency and ensuring high throughput for laboratory environments.
FAQs
-
Q: How does LETIA offer superior sensitivity compared to traditional turbidimetric assays?
A: LETIA achieves superior sensitivity using microscopic latex particles as signal amplifiers. Unlike traditional turbidimetry, where turbidity depends solely on antigen-antibody complex formation, LETIA's inert latex particles significantly increase the mass and size of agglutinated complexes. This amplified aggregation leads to a more pronounced, detectable light scattering change, allowing accurate quantification of analytes at much lower concentrations.
-
Q: Can your LETIA development service accommodate specific analyte targets, even novel ones?
A: Absolutely. Our custom LETIA development accommodates diverse analyte targets, including novel biomarkers. After a detailed consultation, our expertise in strategic antigen/antibody selection and optimized conjugation chemistry ensures high-affinity binding to latex particles. This delivers a highly specific and sensitive assay, even for challenging or newly identified analytes.
-
Q: How do you ensure the long-term stability and shelf-life of the developed LETIA kits?
A: Ensuring long-term stability and shelf-life is paramount. We optimize latex-conjugate stability, select appropriate buffer systems, and incorporate stabilizers. Rigorous, accelerated, and real-time stability studies confirm shelf-life. Our robust quality control throughout manufacturing guarantees consistent quality and integrity of each kit.
-
Q: What kind of automation platforms are your LETIA kits compatible with?
A: Our LETIA kits are broadly compatible with automated clinical chemistry analyzers. During development, we consider diverse high-throughput platform specifications, optimizing reagent presentation, reaction volumes, and measurement wavelengths for seamless integration. This maximizes ease of use and efficiency for our clients.
-
Q: How does LETIA compare to ELISA in terms of speed and throughput for high-volume testing?
A: For high-volume testing, LETIA offers significant advantages over ELISA in speed and throughput. ELISA involves multiple incubation and wash steps, making it time-consuming. In contrast, LETIA is a homogeneous assay, requiring fewer steps and highly amenable to automation. This allows rapid, continuous processing of numerous samples with minimal manual intervention, leading to much higher throughput.
-
Q: What are the key considerations for optimizing latex particle size and surface chemistry in LETIA kit development?
A: Optimizing latex particle size and surface chemistry is critical for LETIA kit performance. Particle size impacts light scattering and agglutination rate; smaller particles (e.g., 0.1-0.4 µm) are preferred for turbidimetric signals. Surface chemistry dictates functional groups for covalent antibody/antigen conjugation, influencing coupling efficiency, stability, and minimizing non-specific binding. Careful selection ensures optimal immunoreactivity and assay sensitivity.
In order to evaluate the analytical performance of experimental data, Creative Biolabs offers LETIA-based kits and related development services by our advanced experimental facilities and automatic platforms. Commercial kits can be applied in IVD fields for determining the diagnosis upon different medical conditions and promoting basic biological research and clinical practice. For more detailed information, please feel free to contact us.
References
-
Daly, Marie-Louise, et al. "A 58-year-old woman with abdominal symptoms and elevated C-reactive protein." PLoS Medicine 5.7 (2008): e149.
-
Xia, Yanyan, et al. "A sensitive three monoclonal antibodies based automatic latex particle-enhanced turbidimetric immunoassay for Golgi protein 73 detection." Scientific Reports 7.1 (2017): 40090.
-
Wey, Leoni, et al. "Bioinformatical design and performance evaluation of a nucleocapsid-and an RBD-based particle enhanced turbidimetric immunoassay (PETIA) to quantify the wild type and variants of concern-derived immunoreactivity of SARS-CoV-2." Biomedicines 11.1 (2023): 160.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.

 Fig.1 Schematic illustrating one possible mechanism of how an antibody interferes with particle-enhanced turbidimetric immunoassays.1,4
Fig.1 Schematic illustrating one possible mechanism of how an antibody interferes with particle-enhanced turbidimetric immunoassays.1,4
 Fig.2 Linearity of LETIA/LTIA.2,4
Fig.2 Linearity of LETIA/LTIA.2,4
 Fig.3 Evaluation of variant cross-reactivity via S-RBD-based PETIA.3,4
Fig.3 Evaluation of variant cross-reactivity via S-RBD-based PETIA.3,4

