Recent advancements in molecular biology greatly facilitated the production of antibody fragments, in particular single-domain antibody (sdAb) and their application as a probe in molecular imaging and immunodetection systems. Armed with advanced phage display technology, Creative Biolabs provides an integrated solution for custom recombinant sdAb selection and validation. With the increasing demand for molecular diagnostics and in vivo imaging, Creative Biolabs fully integrates existing resources and leverages our technical experience in antibody preparation and disease diagnosis to provide our customers with sdAb-based diagnostic tools development services for academic use.
SdAbs are recombinant, single-domain, variable fragments of camelid heavy chain-only antibodies with an approximate molecular weight of 12-15 kDa. SdAbs are highly adaptable tools for disease research as they enable specific modulation of targets, enzymatic and non-enzymatic proteins alike. For instance, in tumor research, molecular imaging studies benefit from the rapid, homogeneous tumor accumulation of sdAbs and their fast blood clearance, permitting previously unattainable fast tumor visualization.
 Fig.1 A schematic representation of sdAb. Distributed under Public Domain, from Wiki, without modification.
Fig.1 A schematic representation of sdAb. Distributed under Public Domain, from Wiki, without modification.
Detection probes should ideally meet most of the following characteristics: high probe accessibility, stability and selectivity towards the antigen, and large-scale production in a cost-effective manner. The superior characteristics of sdAb made it possible to obtain a higher probe density and the facile engineering of the sdAb allowed a directional immobilization, so that a higher ligand binding capacity is obtained without binding interference from contaminants in the sample. For affinity chromatography, coupling green fluorescent protein (GFP)-binding sdAbs onto a monovalent matrix allows fast, efficient, one-step isolation of GFP-fusion proteins and their interacting partners. Besides, sdAbs can be generated to uncover novel, species-specific epitopes and that such sdAbs are well suited to develop species-specific antigen detection assays.
Molecular imaging is the foundation for an accurate diagnosis. Recently, sdAbs have attracted much attention as they fulfill most of the requisites of an ideal probe for successful molecular imaging. Among different imaging techniques, single photon emission computed tomography (SPECT), positron emission tomography (PET), ultrasonography (US), magnetic resonance imaging (MRI), and optical imaging have been successfully employed for molecular imaging using sdAbs. As an intriguing platform, sdAbs have been proposed as potential molecular imaging tools not only in oncology but also for monitoring and quantifying arthritis, atherosclerosis and other inflammatory diseases. In previous studies, sdAbs based imaging tools against EGFR, HER-2, HGF, MMR, VCAM-1, CEA have been developed for pre-clinical and/or clinical molecular imaging.

If you are interested in our services, please feel free to contact us for more information.
1. Robust Single-Domain Antibodies for Sensitive Bacillus anthracis Detection
 Fig.2 Specificity of sdAbs for Bacillus species spores.1,3
Fig.2 Specificity of sdAbs for Bacillus species spores.1,3
To address the limitations of current assays for Bacillus anthracis, this study isolated a series of sdAbs from a phage display library created using immunized llamas. Six sdAb families were characterized for target specificity, affinity, and thermal stability following selection against bacterial spores. All six families targeted the S-layer protein EA1, found in Bacillus anthracis spores and vegetative cells. The sdAbs showed high specificity for Bacillus anthracis and its spores, with nanomolar affinity. Additionally, the sdAbs retained functionality after undergoing multiple cycles of thermal denaturation and refolding. This research highlights the potential of these sdAbs for improving current assays and biosensors, demonstrating their robustness and applicability in detecting Bacillus anthracis.
2. Engineering a HER2-Targeting Single-Domain Antibody for Diagnostic Applications
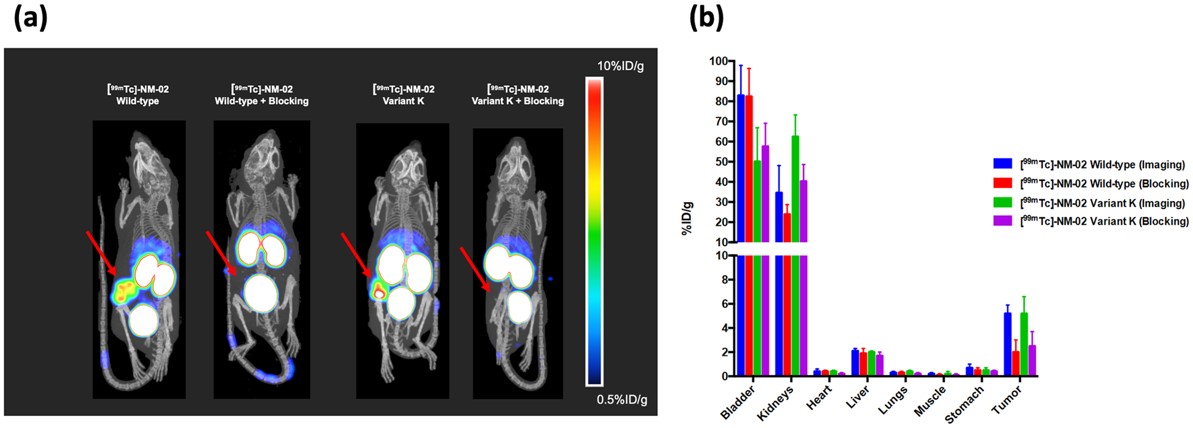 Fig.3 SPECT/CT imaging of humanized anti-HER2 sdAb.2,3
Fig.3 SPECT/CT imaging of humanized anti-HER2 sdAb.2,3
This study identified a highly specific anti-HER2 (human epidermal growth factor receptor-2) sdAb as a valuable theranostic agent. X-ray crystallography revealed a unique epitope distinct from current anti-HER2 antibodies. To minimize immunogenicity, the sdAb was humanized while preserving binding properties, with a dissociation constant of 212 pM, nearly the sdAb. Full developability profiling showed favorable thermal and physical biochemical properties, indicating its suitability for development. The lead humanized anti-HER2 sdAb (variant K) was successfully applied in HER2-specific imaging in breast cancer HER2+/BT474 xenograft mice, demonstrating its potential as an imaging tool in vivo.
References
For Research Use Only.
