The emergence of antibody-drug conjugates (ADCs) as a potential cancer treatment pathway has attracted a great deal of attention. By combining the selective specificity of monoclonal antibodies with the cytotoxicity of drug molecules, the therapeutic index is improved, and cancer cells are selectively targeted while minimizing systemic toxicity.
There are several challenges, including selecting the right target antigen, enhancing antibodies, linkers, and payloads, as well as drug resistance mechanisms and side effect management. However, we can also use site-specific coupling techniques, new antibody formats, and combination therapy strategies to overcome these obstacles.
- ADC drugs approved by the FDA
As of 2023, the U.S. Food and Drug Administration (FDA) has approved 15 different antibody-drug conjugates (ADCs) targeting a variety of antigens for cancer treatment. These drugs target specific antigens such as CD33, CD30, HER2, CD22, CD79b, B-cell maturation antigen (BCMA), Nectin-4, Tissue Factor (TF), EGFR, and folate receptor alpha (FRα). Some of these antigens are targeted by multiple ADCs, demonstrating the critical focus on certain cancer markers.
- ADC drug composition
ADC drug typically consists of a monoclonal antibody, cytotoxic payload, and linker molecule.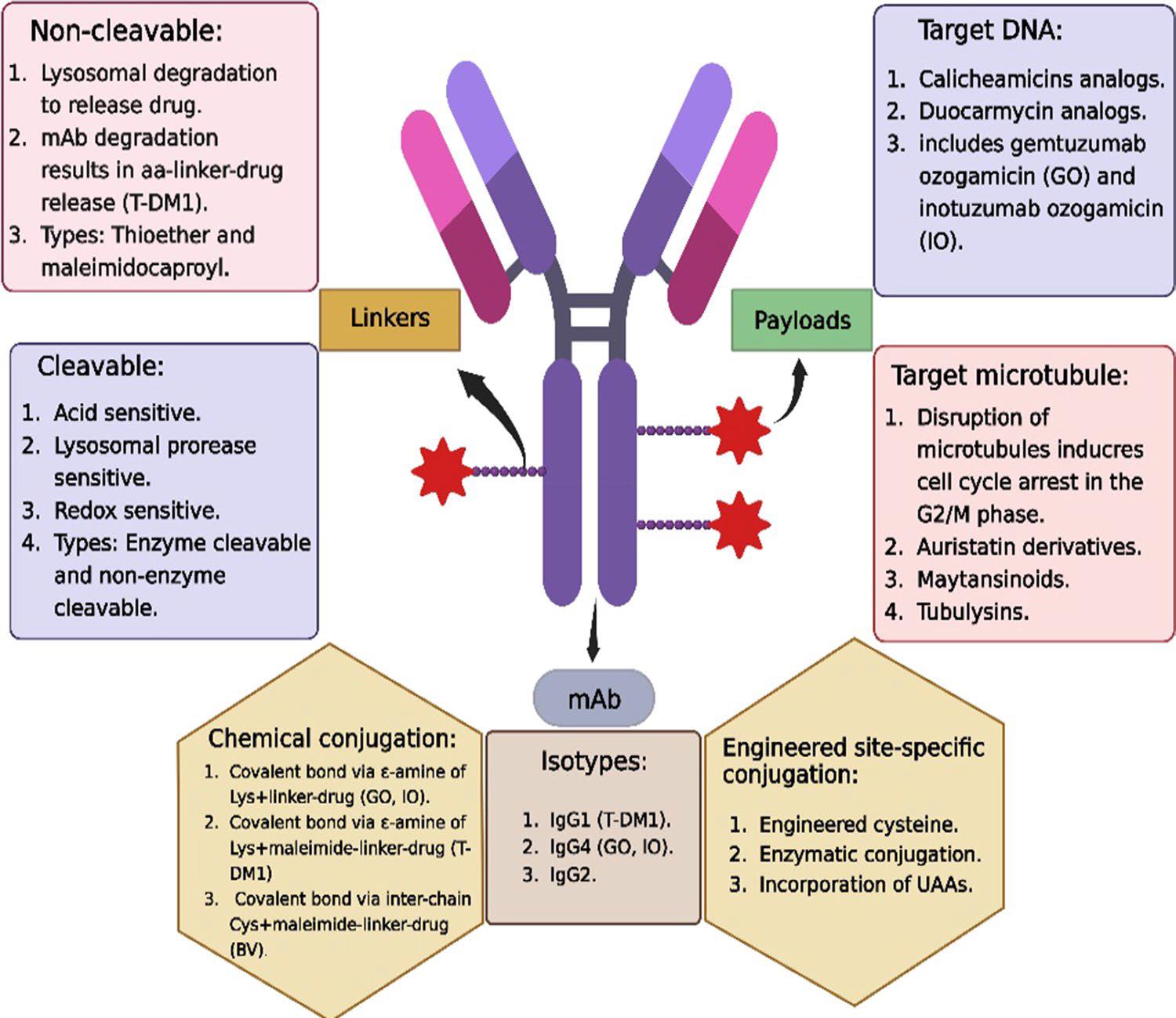
Fig. 1 ADC Structure.1
See cytotoxic payload products we provide
| Catalog | Product name | CAS NO | Purity |
| ADC-P-004 | Aurstatin E | 160800-57-7 | 95% |
| ADC-P-005 | Auristatin F | 163768-50-1 | 95% |
| ADC-P-012 | Dolastatin 10 | 110417-88-4 | 95% |
| ADC-P-015 | Maytansinol | 57103-68-1 | 95% |
| ADC-P-018 | MMAD | 203849-91-6 | 95% |
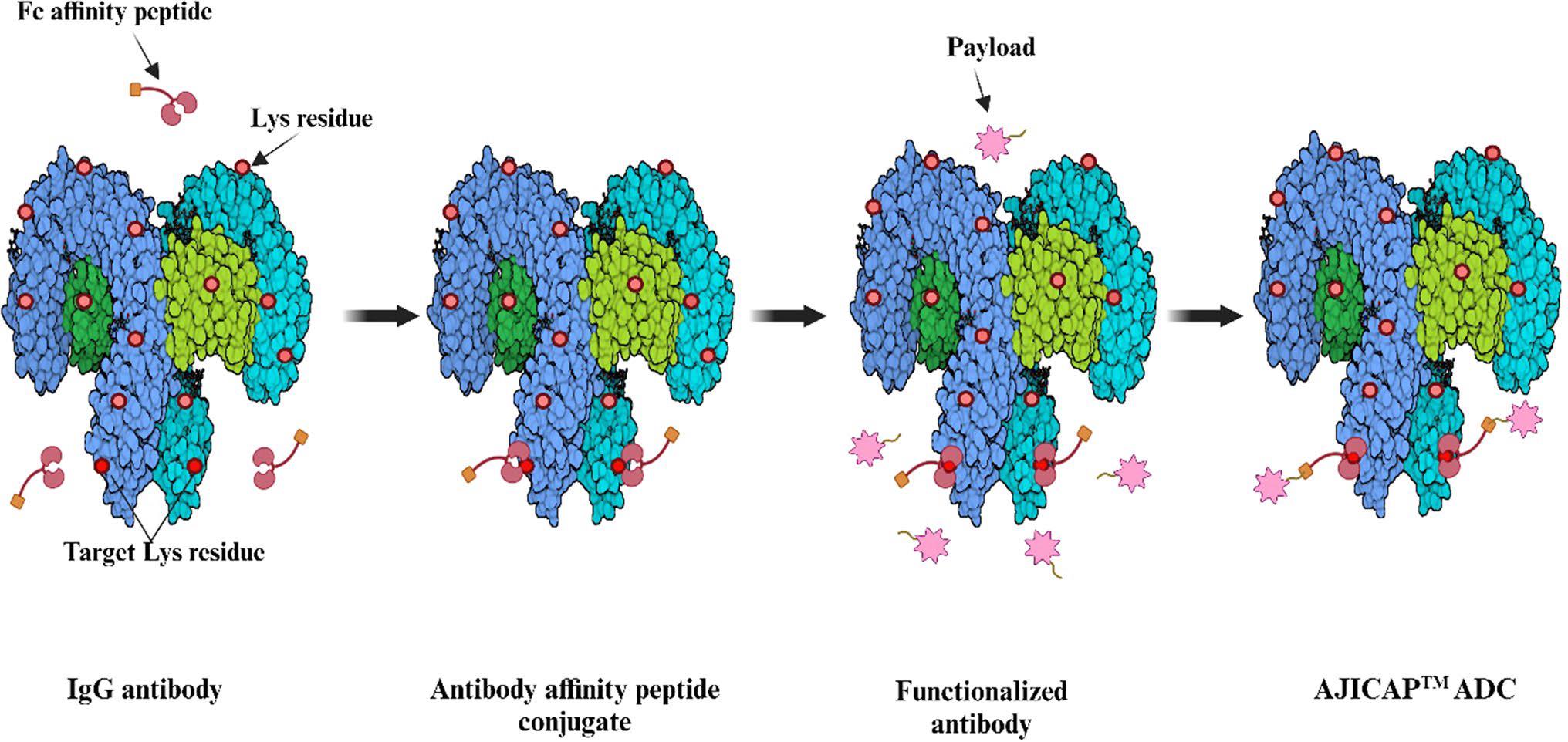
- ADC is designed step-by-step using AJICAP technology
AJICAP technology can modify natural IgG antibodies to create ADCs. The steps include affinity peptide attachment to a lysine residue, antibody functionalization at the peptide binding site, and drug molecule conjugation. This enables targeted drug delivery and precision medicine applications.
Fig. 2 The step of creating ADCs using the AJICAP technology.1
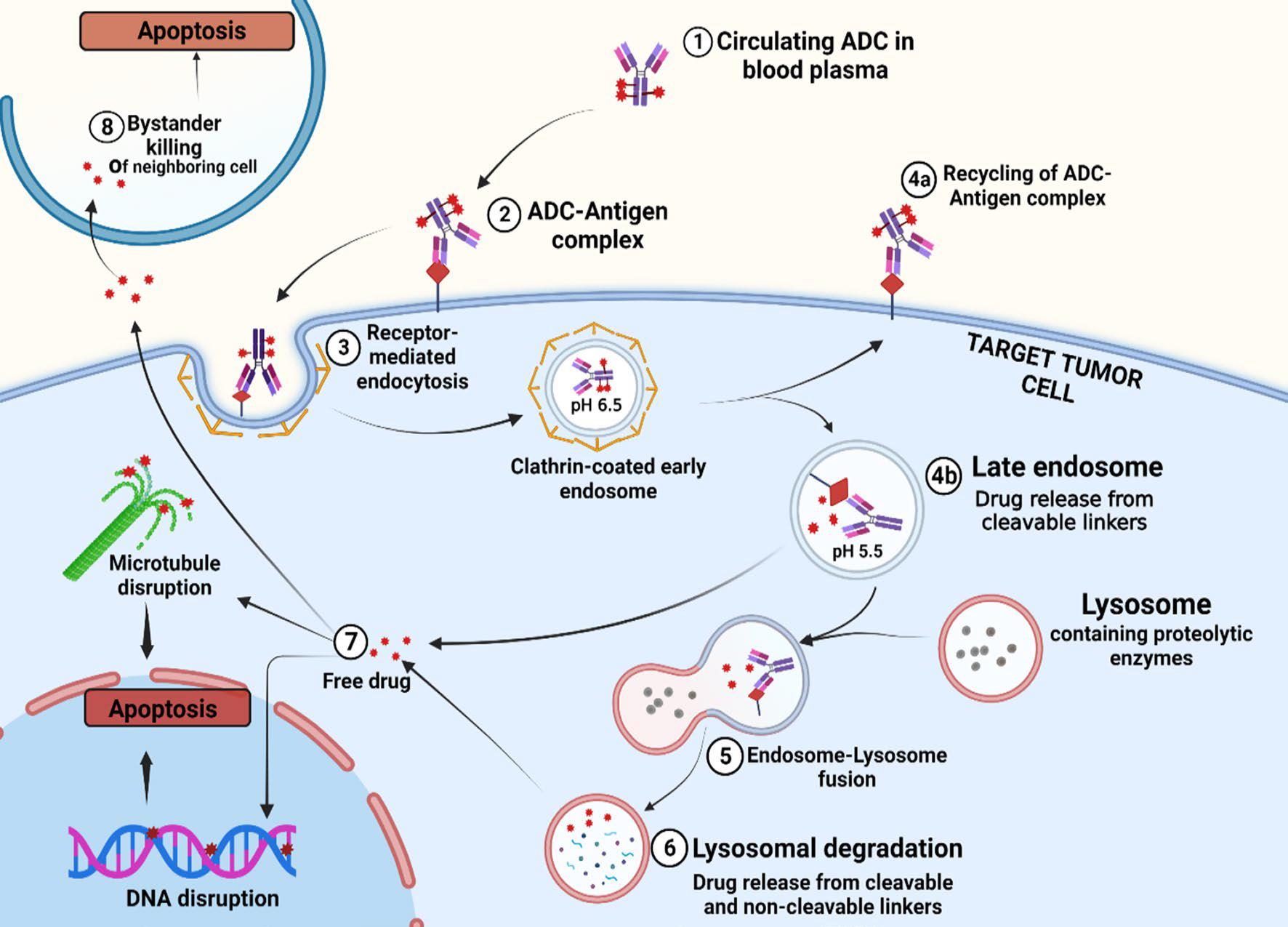
- A general description of the mode of action of an antibody-drug conjugate
The mechanism of ADC drugs consists of several stages. First, the ADC attaches to a specific antigen in the cancer cells. This interaction causes receptor-mediated endocytosis, in which the intact ADC antigen complex is internalized by cancer cells. Some ADC-antigen complexes are recycled back to the cell surface, while the drug remains in the endonuclear body. If this complex follows the drug release pathway, endosomes mature into late endosomes, resulting in the degradation of cleavable linkers and the release of cytotoxic loads. The anaphase nucleosomes then fuse with lysosomes, and the ADC is broken down by lysosomal enzymes, which release cytotoxic drugs. In addition, free drugs may also have an effect on neighboring cancer cells, causing a bystander effect.
Fig. 3 Mode of action of ADC.1
- Mechanisms of ADC resistance in HER2-positive breast cancer
Resistance mechanisms in breast cancer to ADCs include decreased antigen expression, impairments in antigen-ADC complex internalization, defective lysosomal degradation hindering payload release, and drug transporters eliminating payloads before cytotoxic effects initiate. These mechanisms reduce ADC efficacy, contributing to resistance.
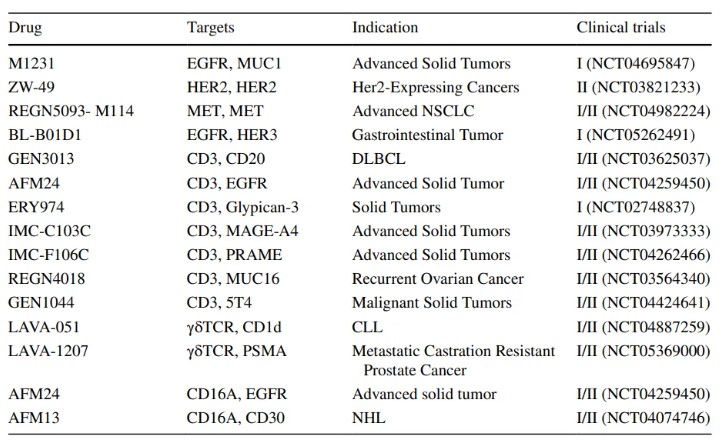
- Clinical trials of bispecific ADCs
As our understanding of the molecular mechanisms that drive cancer progression continues to expand, we look forward to the development of new ADCs that provide more effective and personalized treatment options for cancer patients.
Reference
- Kumari, Shivangi, et al. “Antibody-drug conjugates in cancer therapy: innovations, challenges, and future directions.” Archives of Pharmacal Research 47.1 (2024): 40-65.