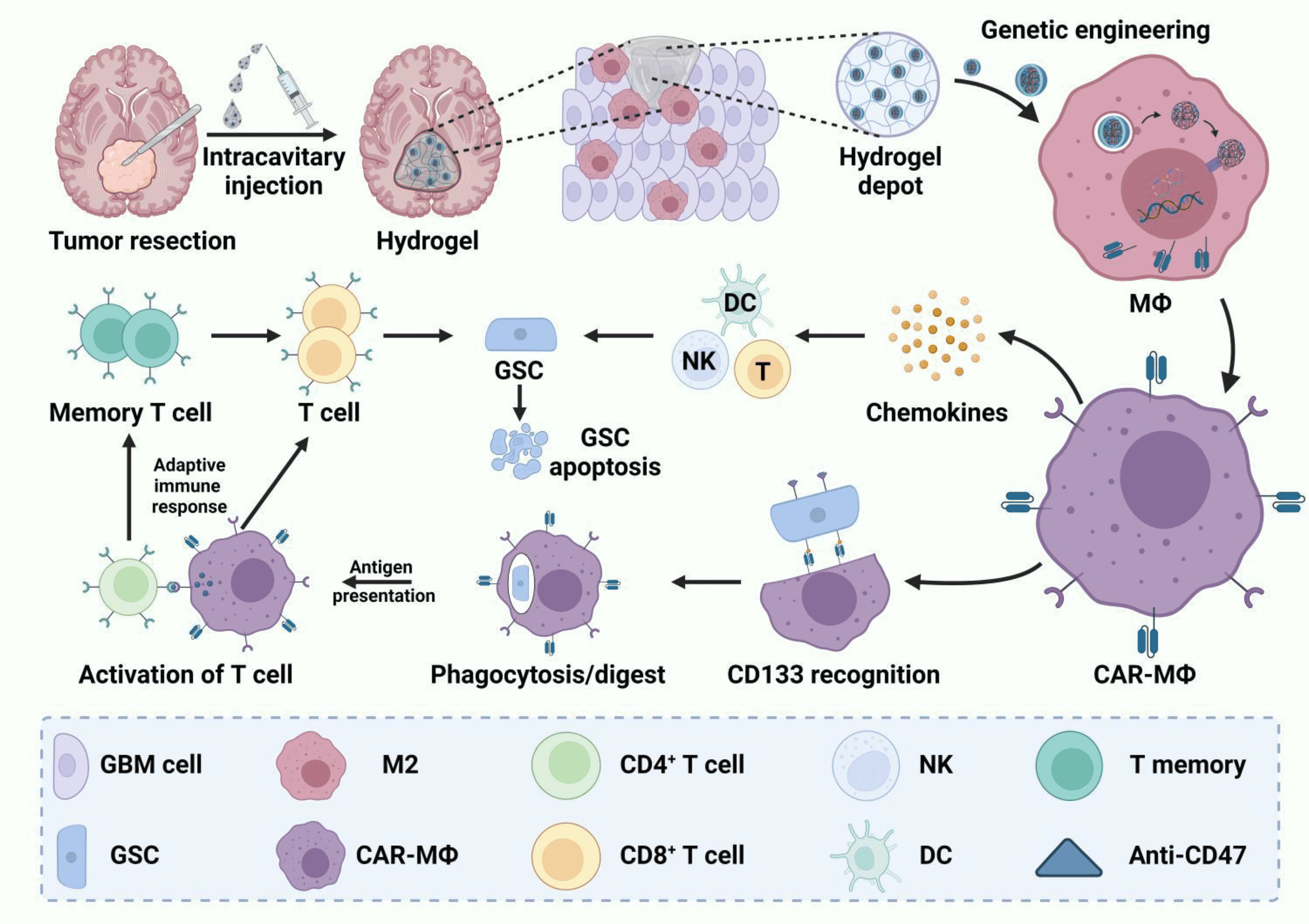
In recent years, Chimeric Antigen Receptor (CAR) technology has made significant progress in the treatment of hematologic malignancies, particularly in acute lymphoblastic leukemia (ALL), lymphoma, and plasma cell myeloma (PCM). However, the effectiveness of CAR-T cell therapy in treating solid tumors remains suboptimal. This has prompted researchers to explore alternative immunotherapy methods to overcome the major challenges faced by current CAR-T cell therapies. Macrophages, with their strong phagocytic ability, antigen-presenting function, and ability to modulate the tumor microenvironment and stimulate adaptive responses, have emerged as a promising option.
CAR-M therapy (CAR-Macrophage therapy) leverages the properties of macrophages, endowing them with specific antitumor abilities through genetic engineering. Macrophages play key roles in tumors by phagocytosing cancer cells, secreting cytokines and chemokines, infiltrating dense tissues, and accumulating in tumors. These characteristics make macrophages an operable candidate in CAR immunotherapy. Since Biglari et al. first engineered CEA-targeted CAR molecules into human monocytes in 2006, the development and optimization of CAR-macrophages have been ongoing. Currently, two CAR-M therapies (CT-0508 and MCY-M11) have received FDA approval to enter clinical trials.
Despite some progress, CAR-M therapy is still in its infancy and faces significant issues, including limited cell resources, resistance to gene transfer, and potential inflammatory pathology. With the combination of human induced pluripotent stem cell (iPSC) preparation, gene editing technologies, and biomaterial delivery technologies, a new generation of CAR-M therapy is expected to possess specific tumor antigen recognition abilities, feasible gene modifications, improved expansion capabilities, and controllable safety. The June 1 issue of Molecular Cancer, “A new era of cancer immunotherapy: combining revolutionary technologies for enhanced CAR-M therapy,” reviewed the latest advancements in CAR-M therapy, covering basic scientific research and clinical trials, and discussed major obstacles hindering the full potential of CAR-M therapy and their solutions.
With the advent of revolutionary technologies such as gene editing, synthetic biology, and biomaterial-supported gene transfer, the integration of these advanced methods will bring about a new generation of CAR-M therapy, enhancing its efficacy, safety, and accessibility. CAR-M therapy not only shows potential in combating hematologic and non-hematologic tumors but also indicates broad future applications in cancer immunotherapy.
In recent years, Chimeric Antigen Receptor (CAR) technology has achieved breakthrough progress in the field of cancer treatment, especially in the treatment of hematologic malignancies. However, the effectiveness of CAR-T cells in treating solid tumors remains limited, prompting researchers to explore new immunotherapy approaches. Macrophages, as the main cells of the innate immune system, have become emerging candidates for CAR therapy due to their multifunctionality and important roles in the tumor microenvironment.
Macrophages are the primary phagocytic cells in the body, capable of engulfing and digesting pathogens such as bacteria, viruses, and cancer cells. Besides their strong phagocytic ability, macrophages have an antigen-presenting function, which can activate adaptive immune responses. Additionally, macrophages play crucial roles in tissue repair, inflammatory responses, and immune regulation. Based on these characteristics, researchers have begun exploring the application of CAR technology to macrophages to develop new cancer immunotherapy methods.
Research Progress in CAR-M Therapy
Basic Principles of CAR-M
CAR is an artificially designed transmembrane receptor typically comprising a single-chain variable fragment (scFv) that recognizes tumor antigens, a hinge domain, a transmembrane domain, and an intracellular signaling domain. By genetically engineering macrophages to introduce CARs, these cells can specifically recognize and kill cancer cells expressing the corresponding antigens.
CARs typically consist of the following parts:
Single-chain Variable Fragment (scFv): The scFv, located on the surface of the CAR cell, connects the heavy chain variable region (VH) and light chain variable region (VL) of the tumor antigen-targeting antibody via a linker sequence.
Hinge Region: The hinge region, usually derived from CD8 or CD28 sequences, provides flexibility to the CAR molecule, enabling appropriate conformational changes when recognizing antigens.
Transmembrane Region: The transmembrane region is responsible for embedding the CAR molecule into the cell membrane and is also typically derived from CD8 or CD28 sequences.
Intracellular Activation Domain: The intracellular activation domain of most CAR molecules consists of CD3ζ and 4-1BB or CD28 regions, responsible for transducing antigen recognition signals into the cell and activating the immune response.
Different Intracellular Activation Domains in CAR-M Design
In CAR-M design, researchers have explored various intracellular activation domains to endow CAR-M with different antitumor capabilities:
Phagocytosis Domains: Integrating phagocytosis domains such as FcRγ, CD3ζ, or Megf10 into CARs enables macrophages to phagocytose antigen-specific target cells and inhibit tumor progression.
PI3K Recruiting Domain: The tandem of FcRγ and the PI3K recruiting domain can achieve whole-cell phagocytosis.
Transmembrane and Intracellular Domain: The transmembrane and intracellular domains of CD147 are used in CAR-M design to secrete matrix metalloproteinases, aiding immune cells in penetrating tumor tissue.
Inflammatory Signaling Domains: Incorporating the intracellular signaling domains of TLR4 or TLR2 into the CAR framework can induce macrophage polarization towards the M1 type, exhibiting antitumor effects.
Costimulatory Domain and Inflammatory Cytokine: A tandem CAR molecule containing the CD28 costimulatory domain, CD3ζ phagocytosis domain, and M1-type cytokine IFN-γ, released through cleavage sites, can inhibit tumor progression through phagocytosis and pro-inflammatory antitumor effects.
Co-expression of CD3ζ and TLR4 Intracellular Domains: Enhances macrophage phagocytosis of target cells and promotes M1 polarization.
Early Research on CAR-M
As early as 2006, Biglari et al. successfully introduced CEA (carcinoembryonic antigen)-targeted CAR molecules into human monocytes, demonstrating the feasibility and safety of this therapy. Since then, researchers have been committed to developing and optimizing CAR-macrophage therapy. Preliminary studies indicate that CAR-M therapy has potential in managing both hematologic and non-hematologic tumors.
Currently, two CAR-M therapies (CT-0508 and MCY-M11) have received FDA approval to enter clinical trials, marking an important step towards the practical clinical application of CAR-M therapy.
Challenges of CAR-M in Solid Tumor Treatment
Despite the potential of CAR-M therapy in treating hematologic malignancies, its application in solid tumors faces many challenges. Firstly, the dense tissue structure and complex tumor microenvironment of solid tumors limit the infiltration and killing effects of CAR-M cells. Secondly, macrophages in the tumor microenvironment are easily “educated” into M2-type macrophages that support tumor growth, weakening their antitumor function. To overcome these problems, researchers are exploring various strategies such as optimizing CAR structure, combining other immunotherapies, and using biomaterials to aid CAR molecule delivery.
Advantages and Potential of CAR-M Therapy
Multifunctionality of Macrophages
Macrophages play multiple roles in the immune system, including phagocytosing pathogens and cancer cells, secreting cytokines and chemokines, and regulating immune responses. These characteristics make macrophages ideal candidates for CAR immunotherapy. Studies have shown that CAR-M cells not only have the ability to directly phagocytose cancer cells but also can activate adaptive immune responses such as T cells through their antigen-presenting function, further enhancing antitumor effects.
Optimization Strategies for CAR-M Therapy
To improve the effectiveness of CAR-M therapy, researchers are continuously optimizing CAR design and the genetic modification of macrophages. For example, introducing different intracellular signaling domains such as CD3ζ and FcRγ can enhance the phagocytic ability and antitumor activity of macrophages. Additionally, combining advanced technologies such as in situ gene editing, synthetic biology, and biomaterial-assisted gene delivery can further enhance the safety and efficacy of CAR-M therapy.
This system is mainly to improve the efficiency of gene editing, enabling these immune cells to efficiently express CAR molecules, thereby enhancing their antitumor activity. LNPs (lipid nanoparticles) are an advanced gene delivery tool whose use in mRNA vaccines (such as COVID-19 vaccines) has garnered widespread attention. The advantages of LNPs include high delivery efficiency, low toxicity, and good biocompatibility. Researchers use LNPs to deliver CAR-encoding mRNA to macrophages and T cells, enabling these cells to specifically recognize and kill tumor cells.
In their research, researchers first optimized the composition of LNPs and the modification of mRNA to improve transfection efficiency in macrophages and T cells. Specifically, they screened a set of lipids and mRNAs and found that incorporating phosphatidylethanolamine (DOPE) into LNPs was crucial for nucleic acid delivery. In vitro experiments showed that this optimized LNP-mRNA system could effectively deliver CAR mRNA to mouse macrophages and human CD8+ T cells, enabling these cells to express CAR molecules and exhibit significant cytotoxic effects, effectively killing B-cell lymphoma cells.
Combining with Other Immunotherapies
In addition to optimizing CAR-M cells themselves, researchers are also exploring combining CAR-M therapy with other immunotherapies to achieve synergistic antitumor effects. For example, combining CAR-M cells with CAR-T cells can leverage the tumor infiltration ability of macrophages and the potent killing ability of T cells, forming complementary advantages. Additionally, combining checkpoint inhibitors such as anti-PD-1 antibody Pembrolizumab (Keytruda) can further release the immunosuppressive tumor microenvironment and enhance therapeutic effects.
Clinical Application Prospects of CAR-M Therapy
As CAR-M therapy continues to develop, an increasing number of clinical trials are underway to evaluate its efficacy and safety in various types of cancer. CT-0508 and MCY-M11 are currently the fastest-progressing CAR-M therapies, both approved by the FDA to enter clinical trials for treating HER2-expressing recurrent or metastatic solid tumors and recurrent/refractory ovarian cancer and peritoneal mesothelioma.
Preliminary clinical data show that CT-0508 exhibits good safety and tolerability in patients, with no significant dose-related toxicity. Additionally, CAR-M therapy shows better tumor suppression effects when delivered locally, providing new insights for future solid tumor treatments.
Despite the immense potential of CAR-M therapy in cancer treatment, its clinical application still faces many challenges. Future research needs to continue optimizing CAR-M cell design, enhancing antitumor efficacy, and reducing potential side effects. Furthermore, exploring more efficient gene delivery systems and combination therapy strategies will help promote the widespread clinical application of CAR-M therapy.
With the continuous advancement of gene editing technology, synthetic biology, and biomaterials science, there is reason to believe that CAR-M therapy will bring new hope and better treatment options for cancer patients in the near future. Through ongoing research and clinical validation, CAR-M therapy is expected to become an important component of cancer immunotherapy, providing a powerful weapon for tackling the challenge of solid tumors.
CAR-macrophage therapy, as an emerging cancer immunotherapy method, has shown great application potential. Despite facing many challenges, through the tireless efforts of researchers and continuous technological advancements, there is reason to believe that CAR-M therapy will play an increasingly important role in future cancer treatment, bringing hope to more patients.
Creative Biolabs masters the most advanced CAR/TCR technology. With state-of-art TCR development platforms and advanced technologies, Creative Biolabs is capable of offering a broad range of CAR-MA therapy development services, from biomarker identification and selection, CAR design and construction, to CAR-MA preparation, and in vitro/in vivo assessments.
Reference
1. Li, Na, et al. “A new era of cancer immunotherapy: combining revolutionary technologies for enhanced CAR-M therapy.” Molecular Cancer 23.1 (2024): 117.