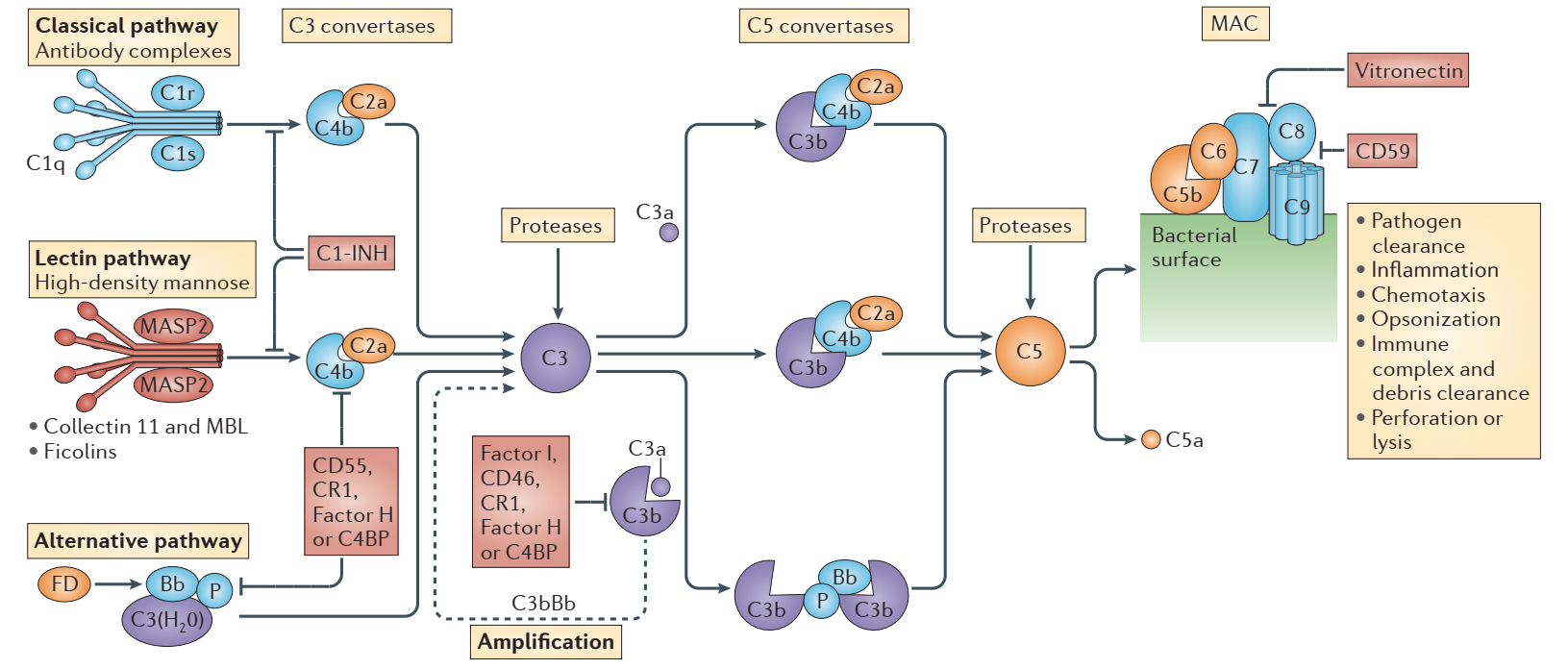
The complement system, also known as the complement cascade, is a crucial component of both the innate and the adaptive immune systems, which can assist or supplement antibodies and phagocytes to clear pathogens and damaged cells and mediate immune bacteriolysis or hemolysis. The complement system is primarily composed of various proteins synthesized by the liver and circulated in blood and tissues, involving approximately 30 kinds of proteins and protein fragments, accounting for about 10% of globulins in serum. The proteases in the complement system cleave specific complement proteins upon activation, releasing cytokines and initiating further cascades. Three biochemical pathways—the classical pathway, the lectin (MLB) pathway, and the alternative pathway—can activate this process. Damage to cells and tissues as a result of abnormal complement system activation can result in autoimmune and inflammatory diseases.
Novel Blockbuster Peptide Pegcetacoplan to Beat Rare Diseases
In the field of complement drugs, giant Alexion has created a blockbuster Soliris (eculizumab), a first-in-class complement C5 monoclonal antibody and the world’s first paroxysmal nocturnal hemoglobinuria (PNH) specific therapeutic drugs. PNH is a rare blood disorder mediated by complement causing chronic intravascular hemolysis, which can be life-threatening and can lead to a mortality rate of up to 35% within five years if left untreated. In 2018, Ultomiris (ravulizumab), a long-acting version of Soliris, was approved. The two drugs are Alexion’s flagship products, with combined sales of more than $5 billion in 2020. At the end of 2020, AstraZeneca announced the acquisition of Alexion for US$39 billion, setting the largest acquisition in the pharmaceutical field that year.
Data show that there are about 15,000 patients with PNH worldwide, which is an extremely rare disease. Before this, the only non-specific treatments for the disease were iron supplementation, glucocorticoids, blood transfusions, and bone marrow transplantation. It was not until Soliris was approved by the FDA in 2007 that PNH patients received the first and only treatment at that time, which was quite pricey. Statistics show that the average patient’s annual treatment cost can reach 678,300 USD, and since lifelong treatment is necessary, the drug is extremely marketable in this extremely rare indication. Soliris was later authorized for additional indications outside of PNH, such as atypical hemolytic uremic syndrome (aHUS), generalized myasthenia gravis (gMG), and neuromyelitis optica spectrum disorder (NMOSD).
However, studies have found that although Soliris is effective in eliminating intravascular hemolysis, most PNH patients treated with Soliris continued to experience mild to moderate extravascular hemolysis-related symptoms because the drug targets C5 downstream of the complement system protein, whereas the upstream C3 protein can still be cleaved, resulting in the deposition of C3d fragments on the surface of red blood cells, and a significant number of red blood cells are intercepted and destroyed by the spleen and liver. Therefore, it is difficult for the patient’s hemoglobin level to return to normal, and most patients still cannot get rid of blood transfusion therapy completely. This issue also leaves opportunities for many latecomers.
Unsurprisingly, a formidable rival to Soliris appeared in 2021. The C3 inhibitor Empaveli (pegcetacoplan) injection from Apellis Pharmaceuticals received FDA approval in May 2021 for the treatment of adult PNH patients. It is the first C3-targeted therapy to be approved to treat adults with treatment-naïve PNH as well as patients switching from the C5 inhibitors Soliris and Ultomiris to Empaveli. This indicates that after almost 15 years, the complement field is now seeing its first “new player”.
For the treatment of PNH, Empaveli is currently approved and available in the US, Saudi Arabia, Australia, as well as the European Union and the United Kingdom under the brand name Aspaveli. Additionally, Apellis has developed a system called ApellisAssist that gives patients access to financial aid tailored to their needs, self-infusion training, product and disease-specific education, and insurance support.
In June 2021, Apellis announced a new drug delivery device under development, with simpler steps, more convenient self-administration, easy portability, alleviating the fear of needles, and improving the patient treatment experience.
Since Pegcetacoplan can be administered intravenously through an ocular injection as well as systemically through a subcutaneous injection, Apellis is evaluating the drug in ophthalmology clinical trials while collaborating with Sobi to investigate its potential applications in hematology, nephrology, and neurology.

Ophthalmology
An intravitreal injection formulation of Pegcetacoplan is being developed for the treatment of geographic atrophy (GA), an advanced form of dry age-related macular degeneration (dAMD) and a leading cause of irreversible blindness worldwide. It affects about 5 million people, and there is currently no specific drug approved. Apellis sees Pegcetacoplan as a “game-changer” for GA patients. Pegcetacoplan can regulate complement activation in the retinal environment, bringing it back to a quiescent state, by blocking the production of C3a and C5a and preventing the accumulation of C3 fragments on retinal cells by inhibiting C3. The FDA has received an NDA application for the medication, which is anticipated to be the first approved treatment for GA.
The FDA has granted Pegcetacoplan fast track designation for the treatment of GA. Apellis declared that it had sent the FDA an NDA application on June 1, 2022. If everything goes according to plan, regulatory approval should come in Q4 2022. In the second half of 2022, Apellis plans to submit an NDA application to the European Drug Administration (EMA), which, meanwhile, will start building European teams and affiliates in Germany and Australia in preparation for potential future approvals and commercialization efforts. It will also create a dedicated ophthalmology team in the US.
Rare Diseases
Paroxysmal nocturnal hemoglobinuria (PNH) is only the first step in Pegcetacoplan’s in-depth deployment in rare diseases. Other indications under development for subcutaneous Pegcetacoplan include C3 glomerulopathy (C3G), immune complex membrane proliferative glomerulonephritis (IC-MPGN), cold agglutinin disease (CAD), hematopoietic stem cell transplantation-related thrombotic microangiopathy (HSCT-TMA), and amyotrophic lateral sclerosis (ALS).
IC-MPGN and C3G are extremely rare kidney diseases, with approximately 5,000 and 8,000 patients in the US and Europe, respectively. The underlying causes and progression of these two diseases are quite similar, including overactivation of the complement cascade, excessive accumulation of C3 breakdown products in the kidneys leading to inflammation and organ damage, and approximately 50% of patients develop renal failure within 5 to 10 years of diagnosis failure, and there is currently no approved treatment. On June 7, 2022, Apellis and Sobi jointly announced that the Phase 3 VALIANT trial of Pegcetacoplan in patients with IC-MPGN and C3G, which assessed Pegcetacoplan’s effectiveness and safety in about 90 patients 12 years of age and older, had finished the first patient dosing.
Elevated C3 levels in the entire motor system of ALS patients may be a factor in chronic neuroinflammation and motor neuron death. In November 2020, Apellis initiated the Phase 2 MERIDIAN trial to evaluate the safety and efficacy of Pegcetacoplan in patients with ALS. The trial completed patient enrollment in March 2022, enrolling approximately 250 adults with ALS, with top-line data expected in mid-2023.
CAD is a serious, chronic, rare blood disorder affecting approximately 10,500 people in the US and Europe, and patients may suffer from chronic anemia and an increased risk of thrombosis, such as stroke. A rare blood disorder called HSCT-TMA may be a fatal side effect of bone marrow transplantation. Research on Pegcetacoplan for CAD and HSCT-TMA indications is currently led by Sobi. In early 2022, the Phase 2 trial of Pegcetacoplan in HSCT-TMA patients completed the first patient dosing, and Sobi expects to initiate a Phase 3 trial of the drug in CAD patients in the second quarter of 2022.
Additionally, Apellis is working with professional and academic researchers to assess the potential of Pegcetacoplan delivery via an adeno-associated virus (AAV) vector for gene therapy.
Disclaimer: Creative Biolabs focuses on promoting biological and biomedical research globally. This article is for information exchange purposes only. This article is also not a treatment plan recommendation. For guidance on treatment options, please visit a regular hospital.
