Background Activation of CP Services Overview Related Products Published Data Protocols Q&A Resources
What is the Classical Pathway?
The classical pathway is one of three activation pathways of the complement system, which is a major contributor to
the defense of infections, clearance of pathogens, removal of apoptotic/necrotic cells, and maintenance of
homeostasis. There are at least 21 different serum proteins have been confirmed as components of the classical
pathway, in which 11 major protein play the most critical role, including C1q, C1r, C1s, C2, C3, C4, C5, C6, C7, C8,
C9. In immune responses, the antibody isotypes IgG or IgM specifically recognizes invasive antigens to form the
antigen-antibody complexes, which is the initiation factor of the classical complement pathway. C1q, as part of C1
complex (C1q C1r2C1s2), has affinity for the Fc regions of IgG/ IgM. It is the first enzyme in this pathway to
trigger a series of enzymatic events. The classical pathway can also be activated by apoptotic/necrotic cells and
acute phase proteins.
 Fig. 1 Illustration of the classical and alternative pathway. Distributed under Public Domain, from Wiki, without modification.
Fig. 1 Illustration of the classical and alternative pathway. Distributed under Public Domain, from Wiki, without modification.
Activation of the Classical Pathway
The enzyme cascade can be divided into three phases:
-
Initiation
The recognition of C1q and IgM or hexameric IgG immune complexes, surface proteins of bacteria/viruses, apoptotic
cells, is a signal to start the complement classical pathway. Three steps are showed in this stage:
1) Zymogen C1 binds directly to the triggers via the globular heads of C1q.
2) Ligand binding induces conformational changes in C1q, resulting in subsequent repositioning and autocatalysis of
the C1r zymogen dimer to be active form.
3) C1r cleaves C1s forming fully activated C1s with catalytic capabilities.
-
Formation of C3 convertase
Activated C1s binds C4 and enzymatically liberates C4a and C4b, which in turn promotes the cleavage of C2 into C2a
and C2b. Surface-attached C4b serves as a platform for the formation of C3 convertase (C4bC2a). C3 convertase can
cleave C3 into C3a and C3b, which is essential for the next enzymic reaction.
-
Formation of C5 convertase and MAC
C3b binds to the C4b2a complex to form C5 convertase (C4b2a3b), while C3a plays role in the recruitment of
inflammatory cells (anaphylatoxin). C5 convertase then cleaves C5 into C5a and C5b. C5b combines with other terminal
components C6, C7, C8, and C9 to form the Membrane Attack Complex (MAC), which leads to lysis of invasive bacteria
through insertion into the target cell membranes to create functional pores.
Key Services Offered by Creative Biolabs
Because the classical pathway mediates a lot of autoimmune and inflammatory disorders, the regulation is very strict
to prevent unwanted complement activation. The complement inhibitors which can antagonize excessive activation of
complement have potential clinical application. Scientists have identified a series of inhibitors targeting the
proteins in this pathway, including C1q, C1r, C3 etc.
Creative Biolabs provides a full range of therapeutic antibodies, inhibitors, soluble complement
regulators, as well as customized services based on the classical pathway targets, including:
-
Complement Therapeutic Target-C1 Complex
-
Complement Therapeutic Target-C1 Inhibitor
-
Complement Therapeutic Target-C2
-
Complement Therapeutic Target-C3
-
Complement Therapeutic Target-C4
-
Complement Therapeutic Target-C5
-
Complement Therapeutic Target-C5aR
-
Complement Therapeutic Target-C3 Convertases
-
Complement Therapeutic Target-C5 Convertases
Targeting the classical complement pathway has become a major focus in drug development, especially for conditions
like autoimmune diseases and transplant rejection. Our classical pathway inhibition studies allow researchers to:
-
Screen small molecules, peptides, and antibodies for their ability to inhibit specific components of the
classical pathway
-
Evaluate the efficacy of complement inhibitors in in vitro and in vivo models
-
Study the pharmacokinetics and pharmacodynamics of complement-targeting drugs
At Creative Biolabs, our classical pathway services are designed to enable researchers to study these mechanisms in
detail, offering tools to explore each step of the pathway. One of the fundamental services we offer is complement activation assays, which allow for the detection and
quantification of classical pathway activation in various biological samples. These assays include CH50 assays, C1q
binding assays, and more. Our team of experienced scientists can help you design custom assays, develop new
protocols, and interpret complex data related to complement activation.
Our comprehensive complement platform offers a great number of complement-related products in a rapid and
cost-effective manner. If you are interested, please feel free to contact us for more
details.
Related Product
We understand that each research project is unique, and we are committed to providing personalized solutions to meet
your specific scientific goals. By leveraging our deep expertise and state-of-the-art products, we ensure that your
research is supported by the most reliable and precise tools available.
We offer proteins/peptides, antibodies, kits, inhibitors, sera and plasma used in the study of the classical pathway
of complement activation. You can find the products you need by target.
Browse Products based on target listing:
Published Data
Presented are findings showcased within articles pertaining to classical pathway studies:
1. Classical Pathway Activation Assay
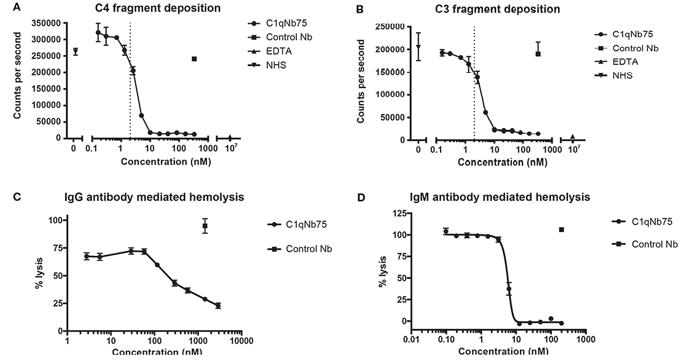 Fig. 2 C1qNb75 inhibits IgG and IgM mediated activation of the classical pathway and hemolysis.1
Fig. 2 C1qNb75 inhibits IgG and IgM mediated activation of the classical pathway and hemolysis.1
Nick S. Laursen et al. described the development and in vitro characterization of a C1q-specific
nanoantibody (C1qNb75) specifically targeting C1q. C1qNb75 binds to the globular head module of human C1q with
sub-nanomolar affinity and prevents classical pathway-mediated IgG and IgM hemolysis. Structural analysis showed
that C1qNb75 recognizes an epitope located predominantly in the C1q B chain that overlaps with the binding sites for
IgG and IgM. Thus, C1qNb75 competitively prevents C1q from binding to IgG and IgM, thereby blocking complement
activation by the classical pathway.
Protocols
Resources
Reference
-
Laursen, Nick S., et al. "Functional and structural characterization of a potent C1q inhibitor targeting the classical pathway of the complement system." Frontiers in immunology 11 (2020): 1504. Distributed under Open Access license CC BY 4.0, without modification.
Questions & Answer
A: The primary role of the classical pathway in the complement system is to deal with pathogens and damaged cells. This pathway becomes active when it recognizes and binds to antibodies that are attached to the surfaces of such cells or pathogens.
A: Creative Biolabs focuses on providing comprehensive complement therapeutic services targeted the classical pathway, with particular interest in drug discovery and development. We offer tailor-made solutions to its clients to meet their specific research needs relating to the classical pathway.The advantages include, but are not limited to, a comprehensive collection of complement therapeutic services, a team of experienced scientists, state-of-the-art techniques and several years of research experience.
A: Complement therapy provides a targeted approach to modulating immune responses by specifically addressing the classical pathway. This can potentially lead to fewer systemic side effects compared to broad immunosuppression. Researchers design complement inhibitors or activators to specifically interact with certain complement proteins in the classical pathway, minimizing off-target effects and enhancing therapeutic specificity.
For Research Use Only.
Related Sections:

 Fig. 1 Illustration of the classical and alternative pathway.
Fig. 1 Illustration of the classical and alternative pathway.  Fig. 2 C1qNb75 inhibits IgG and IgM mediated activation of the classical pathway and hemolysis.1
Fig. 2 C1qNb75 inhibits IgG and IgM mediated activation of the classical pathway and hemolysis.1



