On February 17, 2023, Apellis announced the FDA approval of Pegcetacoplan, a complement C3 inhibitor, as the first molecularly targeted drug for the treatment of dry AMD or geographic atrophy. This approval marks a significant breakthrough in the expansion of complement drugs from rare diseases to common diseases.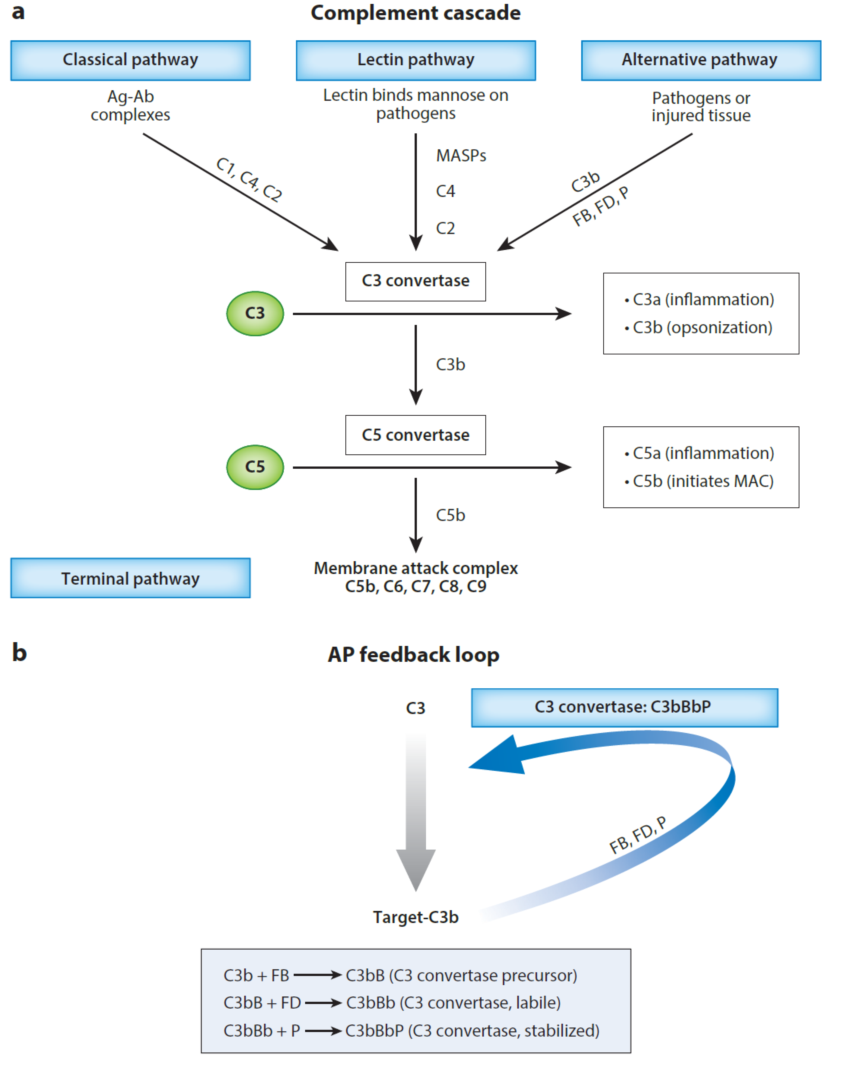
The first complement drug was developed by Alexion, a monoclonal antibody against the C5 target, which was FDA-approved in 2006 for the treatment of the extremely rare disease PNH and subsequently expanded to other rare diseases such as aHUS, gMG, and NMO. The commercial success of Alexion complement drugs has driven many pharmaceutical companies, including major multinational pharmaceutical companies such as Roche, Sanofi, and Novartis, to develop their own pipeline of drugs in the complement field. However, due to the complexity of complement biology and its importance in the human immune system, there have been concerns about the safety risks associated with the use of complement drugs in major, chronic, or common diseases.
After years of clinical exploration based on target selection, molecular structure optimization, and topical application, the successful clinical trial of Apellis for topical application in ophthalmology and the FDA approval of this drug represent the advent of a new era for complement drugs to enter the market of drugs for major, chronic, or common diseases.
Unlike the acquired immune systems mediated by B- and T-cells, the complement system is the primary natural immune system in the body. Its main physiological functions include combating pathogenic microorganisms, removing mutated or necrotic cells from the body, and regulating acquired immunity. The complement system operates through specific or non-specific pattern recognition, initiation of enzymatic cascade reactions, and subsequent activation of C3 and C5 via three main pathways: the classical pathway (CP), which is antibody-mediated, the lectin pathway (LP), which is mediated by recognition of polysaccharide structures, mainly on foreign pathogens or abnormal glycogen structures on the surface of damaged cells, and the alternative pathway (AP), which is activated by natural hydrolysis of complement C3 factor.
During complement activation, the release of inflammatory chemokines such as C3a and C5a recruits immune cells to the site of activation. C3 deposited on the surface promotes phagocytosis through specific receptors on immune cells, while C5b9, or the membrane attack complex (MAC), formed on the membrane surface causes direct cell killing, also known as the terminal pathway (TP). Thus, the complete loss of complement function can lead to severe pathogenic infections and acquired immune abnormalities.
The important physiological functions of complement also define its complex system of functional regulation, which involves dozens of proteins, ranging from blood-soluble proteins to non-receptor or receptor proteins on the surface of the cell membrane, complement activating and complement inhibiting functional proteins, and enzymatically active and non-enzymatically active proteins. Some regulatory proteins also have multiple homologous proteins or mutants generated by multiple different transcript shearing patterns. A perfect regulatory system is an effective guarantee that the complement system will perform its physiological functions.
Abnormalities in complement regulation play a significant role in the development of various human diseases. This results in the abnormal activation of the complement system on the surface of erythrocytes, leading to erythrocyte lysis by the membrane attack complex (MAC) and causing life-threatening symptoms such as haemoglobinuria (erythrocyte lysis), severe anemia (erythrocytopenia), and infection (complement depletion).
One example of such a disease is atypical hemolytic uremic syndrome (aHUS), which is primarily caused by autoantibodies to inhibitory complement factor H or genetic mutations. This leads to abnormal complement activation in the blood and microvascular endothelium, resulting in MAC-mediated damage to vascular endothelial cells, microvascular coagulation (also known as thrombotic microangiopathy or TMA), and renal impairment.
Other diseases caused by complement dysregulation include generalized myasthenia gravis (gMG) caused by autoantibodies to the acetylcholinesterase receptor (AChR), optic nerve myelitis (also known as neuromyelitis optica or NMOSD) caused by autoantibodies to aquaporin-4 (AQP4), and a newly recognized subtype of myelitis optica called neuromyelitis optica spectrum disorder (NMOSD). In all these cases, excessive or sustained activation of the complement pathway at the cell membrane surface by antigen-antibody complexes leads to MAC-mediated destruction and impaired function of the associated cells.
To address these diseases, Alexion has developed Eculizumab, a monoclonal antibody that targets C5 and inhibits the terminal pathway (TP) of the complement system. Eculizumab has been used for the treatment of diseases such as paroxysmal nocturnal hemoglobinuria (PNH), aHUS, gMG, and NMO, as it blocks abnormal complement activation and helps alleviate the symptoms associated with complement dysregulation.
For many years, complement drugs have mainly focused on targeting C5, a well-established therapeutic target for various diseases, including rare conditions like PNH. However, recent developments have expanded the scope of complement-targeting drugs to include other targets as well.
For example, Chugai (Roche) has developed an antibody that binds to a different epitope of Eculizumab, which can be used for patients who do not respond to Eculizumab. This new antibody is administered subcutaneously every four weeks and is set to be the first complement drug to be marketed in China in 2023. Additionally, there are aptamers (Iveric Bio), siRNA (Alnylam), and Alexion targeting C5 that are also in various stages of clinical development.
Furthermore, complement drug development has expanded to targets beyond C5. Apellis has received FDA approval for their C3-targeting, PEG-coupled inhibitory cyclic peptide (pegcetacoplan) for the treatment of PNH in May 2021, as it effectively inhibits extravascular haemolysis (EVH). ChemCentryX’s small molecule inhibitor targeting the C5a receptor (C5aR) was also approved by the FDA in October 2021 for the clinical treatment of autoimmune vasculitis (ANCA), which led to the acquisition of ChemCentryX by Amgen in 2022 for $3.7 billion. Sanofi’s C1s monoclonal antibody was approved by the FDA in 2022 for the clinical treatment of cold agglutinin disease (CAD). Other complement-targeted therapies in clinical development include Omeros Corp.’s MASP-2 and MASP-3 monoclonal antibodies, Novartis’ small molecule inhibitors of complement factor B, Alexion’s small molecule inhibitors of complement factor D, and other protein or gene replacement therapies for complement inhibitory factor H (CFH) or I (CFI). However, most of these complement drug developments for new targets are still based on PNH.
It’s worth noting that abnormal complement activation is not limited to rare diseases like PNH. Complement’s role in the development and progression of immune nephropathies, age-related macular degeneration (AMD), haemorrhagic reperfusion injuries, neurodegenerative diseases, and other major, chronic, or common diseases involving ophthalmology, nephrology, cardiovascular diseases, organ transplantation, and the nervous system has been demonstrated in clinical and preclinical studies. However, due to the limited availability of complement-targeting molecules and preclinical disease models, it remains challenging to assess the safety and efficacy of complement-targeting drugs for these conditions, and the development of complement drugs has been largely limited to rare diseases with few treatment options
Nevertheless, there have been positive developments in expanding complement drugs into larger market indications. For example, Omeros’ MASP-2 monoclonal antibody and Novartis’ CFB small molecule inhibitor have shown significant reductions in proteinuria in Phase II clinical trials for IgAN nephropathy, a larger market with approximately 135,000 patients in the US and one million in China. Apellis’ C3-targeted cyclic peptide and Iveric Bio’s C5-targeted aptamer have filed NDAs with the FDA in 2022 and early 2023, respectively, based on positive results from Phase III trials for dry age-related macular degeneration (dAMD) or geographic atrophy (GA). The FDA approval of Apellis’ products highlights the potential of complement drugs in the market for major, chronic, or common diseases, signaling positive progress in the complement drug market.
We look forward to the approval of more complement-targeted drugs in the coming period to better serve the clinical treatment of more complement-related diseases.
