Bispecific antibody conjugates (BsACs) are a novel antibody therapy strategy that can use two different antigen-binding sites to simultaneously target two different antigens or two different epitopes on the same antigen, thereby achieving multiple interventions and regulation of diseases. BsACs have higher therapeutic effects and lower side effects than monoclonal antibodies (mAbs) and are widely used in tumor immunotherapy and the treatment of other diseases. The concept of BsACs was first proposed by Nisonoff et al. in the 1960s, and then various different types and structures of BsACs appeared with the development of antibody engineering and recombinant DNA technology, such as bispecific IgG, single-arm IgG, bispecific Fv, single-chain Fv, bispecific T cell engagers, etc. BsACs are mainly classified according to their structure, preparation method and function, and currently there are more than 30 mature commercial technology platforms for the production and development of BsACs. Cov-X-Body is a kind of BsAC prepared by CovX technology, which is characterized by connecting a small molecule drug with a bispecific antibody through a cleavable linker peptide, forming a semi-biosynthetic conjugate. CovX technology is a technology based on chemical synthesis and enzyme-catalyzed reactions, which can combine any two small molecule drugs or peptides with any two antibodies, forming conjugates with dual or multiple functions. Cov-X-Body is a new type of BsAC, which has the advantages of simple structure, easy preparation, high stability, low toxicity, etc.
Structural Characteristics of Cov-X-Body
Cov-X-Body is a bispecific antibody conjugate prepared by CovX technology, which has a basic structure of connecting a small molecule drug with a bispecific antibody through a cleavable linker peptide, forming a semi-biosynthetic conjugate. The bispecific antibody part of Cov-X-Body can be any combination of two antibodies, usually using the format of bispecific IgG or single-arm IgG, which have two different antigen-binding sites, and can simultaneously target two different antigens or two different epitopes on the same antigen. The small molecule drug part of Cov-X-Body can be any small molecule compound or peptide with therapeutic activity, usually choosing a drug that has synergistic or complementary effects with the bispecific antibody part to improve the therapeutic effect. The linker peptide part of Cov-X-Body is a water-soluble flexible peptide chain composed of six amino acids, which can undergo hydrolysis reaction under the action of enzymes, thereby releasing the small molecule drug part achieving drug release in the target cells.
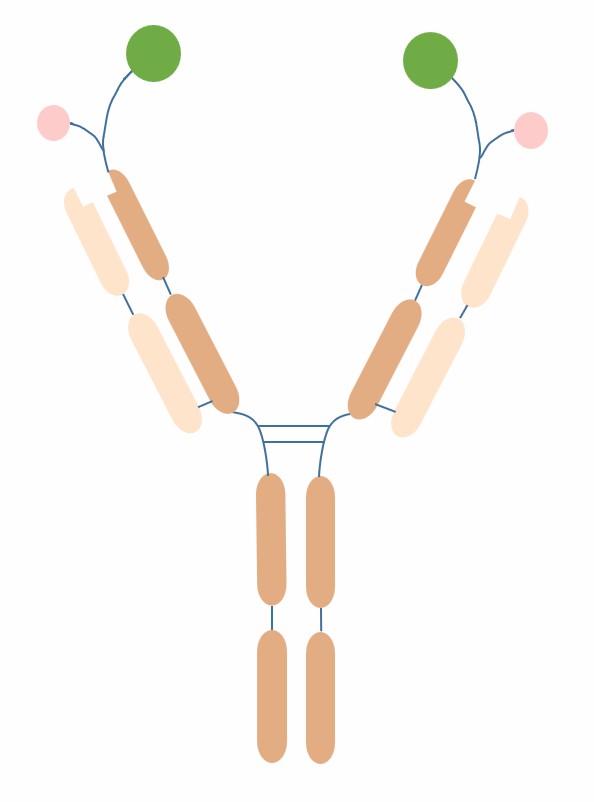
Fig.1 The schematic diagram of the structure of Cov-X-Body (Creative Biolabs)
Clinical Data of Cov-X-Body
Cov-X-Body, as a novel bispecific antibody conjugate, has a few types that have been approved for marketing, mainly for the treatment of malignant tumors such as leukemia, lymphoma and solid tumors.
Table 1. Cov-X-Body approved for marketing
|
Name
|
Targets
|
Approval Time
|
Clinical Efficacy
|
Safety
|
|
Blinatumomab
|
CD19/CD3
|
2014
|
In patients with relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL), compared with standard chemotherapy, it significantly increased the complete remission rate (43% vs. 25%), the minimal residual disease-negative rate (76% vs. 48%), and the median overall survival (7.7 months vs. 4 months).
|
The most common adverse reactions were fever, neurological events, cytokine release syndrome, infection and leukopenia.
|
|
Emicizumab
|
Factor IXa /Factor X
|
2017
|
In congenital hemophilia A patients with factor VIII inhibitors, compared with prophylactic treatment, it significantly reduced the bleeding event rate (87% vs. 22%) and improved their life quality.
|
The most common adverse reactions were injection site reactions, headache, joint pain, and fever.
|
|
Catumaxomab
|
EpCAM/CD3
|
2009
|
In patients with malignant peritoneal effusion (MPE), compared with placebo, it significantly prolonged the time to fluid reaccumulation (46 days vs. 11 days) and increased the survival rate (71% vs. 58%)
|
The most common adverse reactions were fever, nausea, vomiting, diarrhea and abdominal pain.
|
Cov-X-Body in clinical trials mainly targets tumor immunotherapy by activating T cells or NK cells or antagonizing tumor escape mechanisms to achieve tumor cell killing.
Table 2. Cov-X-Body in clinical trials
|
Name
|
Targets
|
Clinical Phase
|
Indications
|
Clinical Efficacy
|
Safety
|
|
Mosunetuzumab
|
CD20/CD3
|
III
|
Relapsed/refractory non-Hodgkin lymphoma (NHL)
|
In Phase I/II trials, the overall response rate was 63.2%, the complete remission rate was 43.4%, the median duration of remission was not reached, and the median overall survival was not reached.
|
The most common adverse reactions were cytokine release syndrome, infection, anemia and leukopenia.
|
|
Epcoritamab
|
CD20/CD3
|
III
|
Relapsed/refractory diffuse large B-cell lymphoma (DLBCL)
|
In Phase I/II trials, the overall response rate was 66%, the complete remission rate was 48%, the median duration of remission was not reached, and the median overall survival was not reached.
|
The most common adverse reactions were cytokine release syndrome, infection, anemia and leukopenia.
|
|
Glofitamab
|
CD20/CD3
|
II
|
Relapsed/refractory NHL and chronic lymphocytic leukemia (CLL)
|
In Phase I trial, the overall response rate was 64.7%, the complete remission rate was 41.2%, the median duration of remission was not reached, and the median overall survival was not reached.
|
The most common adverse reactions were cytokine release syndrome, infection, anemia and leukopenia.
|
|
AMG 596
|
EGFRvIII/CD3
|
I/II
|
Glioblastoma multiforme (GBM)
|
In Phase I trial, some patients showed tumor volume reduction or stabilization, but no statistical significance was achieved.
|
The most common adverse reactions were fever, headache, nausea and vomiting.
|
|
SAR440234
|
CD123/CD3
|
I/II
|
Acute myeloid leukemia (AML) and myeloid malignancies (MMO)
|
In Phase I trial, some patients achieved complete remission or complete remission with incomplete hematologic recovery, but no statistical significance was achieved.
|
The most common adverse reactions were cytokine release syndrome, infection, anemia and leukopenia.
|
|
MGD019
|
PD-1/CTLA-4
|
I/II
|
Solid tumors and lymphomas
|
In Phase I trial, some patients showed tumor volume reduction or stabilization, but no statistical significance was achieved.
|
The most common adverse reactions were rash, diarrhea, nausea and vomiting.
|
References
1. Ma J, et al. Bispecific Antibodies: From Research to Clinical Application. Front Immunol. 2021 May 5;12:626616.
2. Shukla AK, Misra S. Bispecific antibodies and its applications: a novel approach for targeting SARS-Cov-2. J Basic Clin Physiol Pharmacol. 2023 Jan 6;34(1):0068.
3. Nagorsen D, et al. Blinatumomab: a historical perspective. Pharmacol Ther. 2012 Dec;136(3):334–42.
4. Shima M, et al. Factor VIII–Mimetic Function of Humanized Bispecific Antibody in Hemophilia A. N Engl J Med. 2016 May 26;374(21):2044–53.
5. Heiss MM, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer. 2010 Dec 15;127(12):2897–906.
6. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95–106.
7. Irani V, et al. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol Immunol. 2015 Aug;67(1):171–82.
8. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–7.
9. Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature. 1983 Jul 28-Aug 3;304(5923):537–9.
10. Nisonoff A, et al. A univalent hapten-binding derivative of gamma-globulin. Biochem Biophys Res Commun. 1960 Sep;3:81–6.
11. Venkata R Doppalapudi V, et al. Chemical generation of bispecific antibodies. Proc Natl Acad Sci U S A. 2010 Dec 28;107(52):22611–6.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










