In the field of anti-cancer therapy, antibody drug conjugates (ADCs) are a class of chemotherapeutic agents with various advantages, which combine high-affinity and selective monoclonal antibodies (MoAbs) with highly cytotoxic drugs, achieving precise recognition and killing of tumor cells. However, despite some success, ADCs still face many challenges and limitations, most notably improving their therapeutic index. To overcome the limitations of ADCs, a new type of antibody drug conjugate—bispecific antibody conjugates (BsACs)—emerged. BsACs are a class of antibody drug conjugates with two different or identical antigen binding sites, which can simultaneously recognize two different or identical antigens or epitopes. Compared with ADCs, BsACs have stronger targeting ability and a higher cytotoxic drug load, thus improving their therapeutic effect and therapeutic index. Among BsACs, the IgG-IgG format is a common and mature bispecific antibody format, which consists of two different or identical IgG molecules connected by chemical or biological methods. IgG-IgG format BsACs have similar structure, stability, and half-life to IgG and also retain the Fc function of IgG. The IgG-IgG format BsACs have broad application prospects and potential in tumor immunotherapy and other fields.
Structural Characteristics and Generation Methods of IgG-IgG
IgG-IgG format BsACs are a common and mature bispecific antibody format, which consist of two different or identical IgG molecules connected by chemical or biological methods. IgG-IgG format BsACs have similar structure, stability and half-life to IgG and also retain the Fc function of IgG.
The structural characteristics of IgG-IgG format BsACs mainly include the following aspects:
-
Two different or identical IgG molecules: each IgG molecule consists of a heavy chain and a light chain, which are connected by disulfide bonds, forming a Fab region and an Fc region.
-
Two different or identical antigen binding sites: each antigen binding site consists of a heavy chain variable region (VH) and a light chain variable region (VL), which interact with each other by hydrogen bonds and van der Waals forces, forming an antigen recognition structure.
-
Two different or identical Fc regions: each Fc region consists of two heavy chain constant regions (CH2 and CH3), which are connected by disulfide bonds, forming a structure with Fc function.
-
The connection mode between the two IgG molecules can be chemical or biological: chemical connection usually uses free thiol groups or other functional groups on the heavy chain constant region (CH3) to cross-link, and biological connection usually uses heavy chain heterologous recombination or light chain matching techniques to recombine.
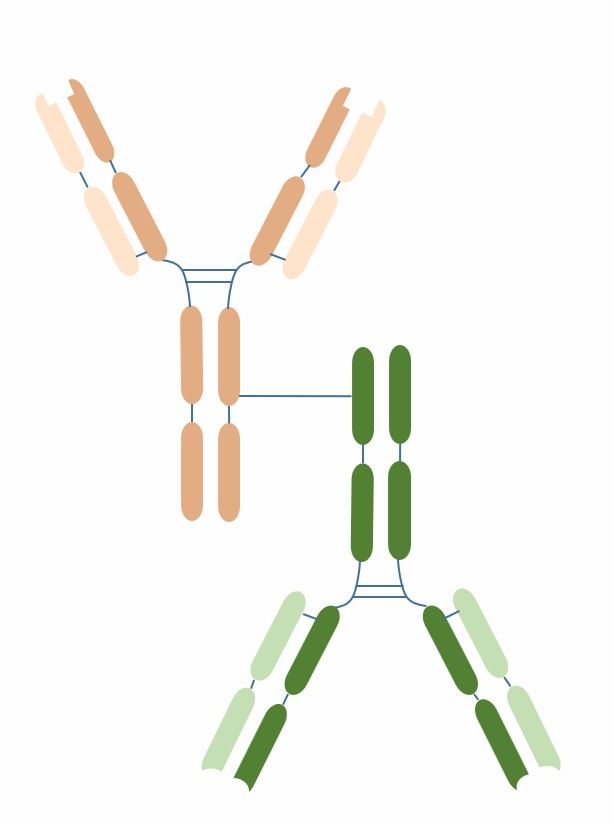
Fig.1 The schematic diagram of the structure of IgG-IgG (Creative Biolabs)
The generation methods of IgG-IgG format BsACs mainly include the chemical connection method, the heavy chain heterologous recombination method, and the light chain matching method. Chemical connection method, which uses chemical reagents to cross-link two different or identical IgG molecules through free thiol groups or other functional groups on the CH3 region, forming bispecific antibodies. This method is simple and fast, but may lead to problems such as product heterogeneity, poor stability, high immunogenicity, etc. Heavy chain heterologous recombination method, which uses genetic engineering techniques to recombine the heavy chain genes of two different or identical IgG molecules heterologously, forming a mixed DNA fragment containing two different or identical heavy chains, and transfecting it into mammalian cells to express bispecific antibodies. This method can produce uniform, stable and functional bispecific antibodies, but requires optimization of parameters such as heavy chain pairing and light chain selection. Light chain matching method, which uses genetic engineering techniques to match the light chain genes of two different or identical IgG molecules so that each light chain can only pair with one heavy chain, and transfecting it into mammalian cells to express bispecific antibodies. This method can avoid the loss or reduction of function caused by light chain mismatching but requires screening of suitable light chain sequences.
Clinical Applications of IgG-IgG
The clinical applications of IgG-IgG type BsAbs are mainly focused on tumor immunotherapy, by targeting tumor-associated antigens and immune effector cells, or by targeting two tumor-associated antigens, to enhance the killing of tumor cells and inhibit the growth and metastasis of tumor cells. So far, three IgG-IgG type BsAbs have received market approval, namely catumaxomab, blinatumomab, and emapalumab.
Table 1. IgG-IgG approved for marketing
|
Name
|
Target
|
Approval
|
Indication
|
Population
|
Country/Region
|
|
catumaxomab
|
EpCAM/CD3
|
2009
|
Malignant ascites
|
Patients with EpCAM-positive tumors
|
EU, India, etc.
|
|
blinatumomab
|
CD19/CD3
|
2014
|
B-cell precursor acute lymphoblastic leukemia (ALL)
|
Patients with relapsed or refractory disease or minimal residual disease (MRD) positive disease
|
USA, EU, etc.
|
|
emapalumab
|
IFNγ/CD3
|
2018
|
Primary HLH
|
Patients refractory or intolerant to conventional therapy
|
USA
|
In addition, there are many IgG-IgG type BsAbs in different stages of clinical trials, such as zolbetuximab, RG7802, pasotuxizumab, and so on.
Table 2. IgG-IgG in clinical trials
|
Name
|
Target
|
Phase
|
Indication
|
Population
|
Country/Region
|
|
zolbetuximab
|
CEA/CD3
|
III
|
Advanced gastric or esophageal cancer
|
Patients with CEA-positive tumors
|
USA, EU, Japan, etc.
|
|
RG7802
|
CEA/CD3
|
II
|
Metastatic colorectal cancer
|
Patients with CEA-positive tumors
|
USA, EU, etc.
|
|
pasotuxizumab
|
PSMA/CD3
|
II
|
Metastatic castration-resistant prostate cancer (mCRPC)
|
Patients with PSMA-positive tumors
|
USA, EU, etc.
|
References
1. Ma J, et al. Bispecific Antibodies: From Research to Clinical Application. Front Immunol. 2021 May 5;12:626616.
2. Comer F, et al. Bispecific and Biparatopic Antibody Drug Conjugates. In: Ducry L, ed. Innovations for Next-Generation Antibody-Drug Conjugates. Cancer Drug Discovery and Development. Springer; 2018:217-240.
3. Zhao Q. Bispecific Antibodies for Autoimmune and Inflammatory Diseases: Clinical Progress to Date. BioDrugs. 2020 Feb;34(1):111-119.
4. Esfandiari A, et al. Bispecific antibodies in oncology. Nat Rev Drug Discov. 2022 Jan 28;21(1):11-12.
5. Kontermann RE, et al. Bispecific antibodies. Drug Discov Today. 2015 Jun;20(7):838-847.
6. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
7. Fan G, et al. Bispecific antibodies and their applications in tumor immunotherapy. Cell Mol Immunol. 2015 Jan;12(1):24-33.
8. Labrijn AF, et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019 Aug;18(8):585 -608.
9. Bacac M, et al. Bispecific antibody formats for cancer therapy. Curr Opin Biotechnol. 2020 Dec;65:49 -57.
10. Wu J, et al. BsAb-engineered NK cells enhance immunotherapy for EGFR-positive malignancies via dual-targeting activation and PD-L1 checkpoint blockade. Clin Cancer Res. 2018 Apr 15;24(8):2003 -2014.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










