Creative Biolabs is one of the well-recognized experts who are professional in in vitro diagnostics (IVD) antibody development against various markers. Now we are glad to provide the custom IVD recombinant antibody discovery service for our customers all over the world.
The recombinant antibody is antibody fragments encoded by recombinant coding genes. Compared with classical antibodies, recombinant antibodies present a series of advantages in both medical and research applications which including rapid tissue penetration, ease of engineering and manufacture, broader biodistribution, and rapid clearance from blood. Moreover, we can control the antibody type by controlling the DNA sequence. In general, the recombinant antibody contains Fv, Fab, Fc, scFv, and sdAb.
 Fig.1 Production of the recombinant antibody.1,5
Fig.1 Production of the recombinant antibody.1,5
Affinity maturation is the process to improve antibody affinity for an antigen both in vivo and in vitro. With extensive experience, now we can provide two antibody affinity maturation methods, untargeted mutagenesis, and oligonucleotide-directed mutagenesis, for the construction of defined or random sub-libraries to introduce a large number of mutants of the original antibody. The subsequent stage is library screening, here two screening strategies are available which are “surface-panning” strategy and “solution-sorting” strategy. Moreover, we can also provide the antibody affinity measurement service.
Antibody reformatting is the process to obtain soluble antibody fragments or full monoclonal antibodies from the antigen binding regions of an antibody. Apart from the Fc portion, the reformatting of variable region results in a variety of different fragments such as scFv, Fab, sdAb, and BsAb. What's more, we can even re-engineer the antibodies into virtually any format to meet our clients' special needs.
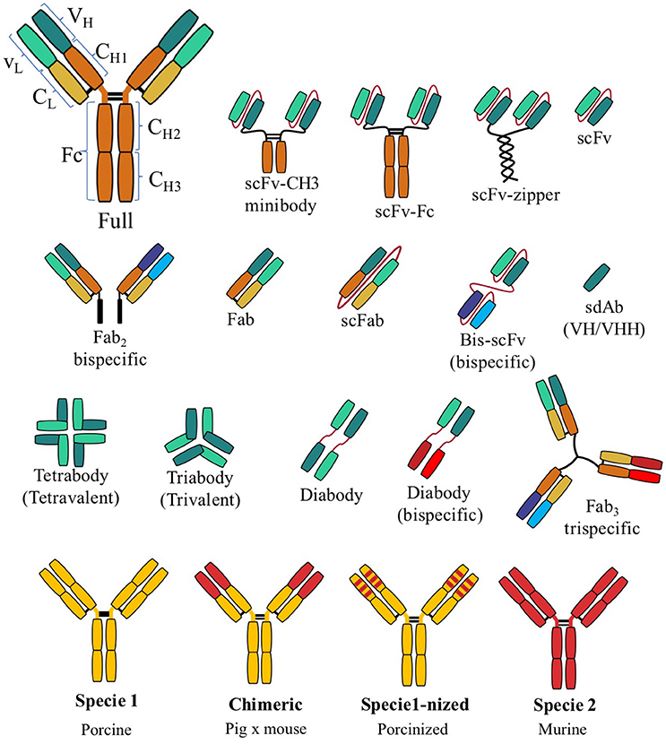 Fig. 2 Common types of recombinant antibody.2,5
Fig. 2 Common types of recombinant antibody.2,5
Based on our extensive experience and advanced platform, Creative Biolabs is proud to provide the IVD recombinant antibody development services for our customers all over the world. Our service can be tailor-designed to meet your specific needs. If you are interested in our services, please do not hesitate to contact us for more details.
1. Recombinant scFv Antibody Targeting Platelets Produced in Pichia pastoris
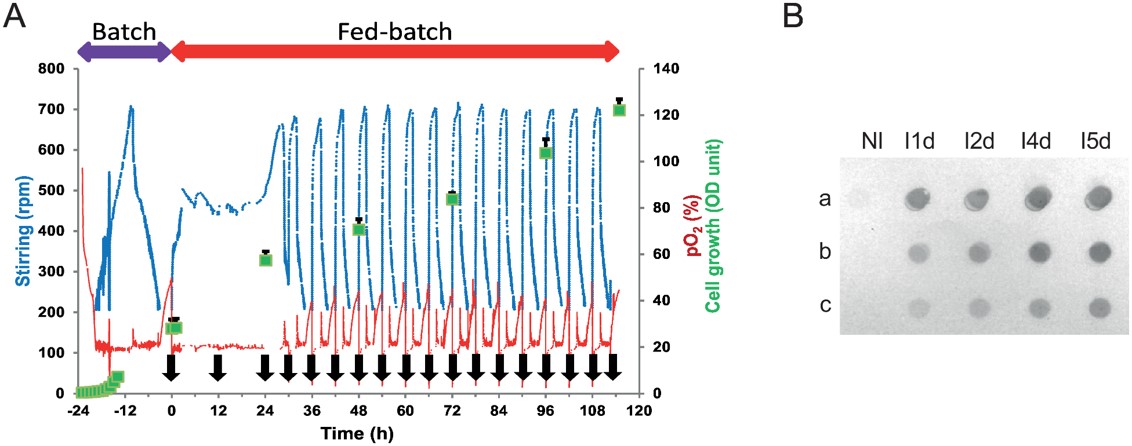 Fig.3 The anti-αIIbβ3 scFv production process.3,5
Fig.3 The anti-αIIbβ3 scFv production process.3,5
In this study, researchers successfully produced highly purified and biologically active scFv fragments in a yeast cell expression modality, obtained from a human anti-αIIbβ3 antibody (HuAb), particularly designed to target atheromatous lesions associated with platelet presence. Various methods, including ELISA, flow cytometry with platelets, affinity binding assays, and immunohistochemistry on atherosclerotic plaques from both animal models and human coronary tissue samples were used to evaluate the immunoreactivity of the anti-αIIbβ3 scFv. Additionally, the optimized expression conditions enabled the recovery of scFv fragments in a form that was not only highly purified but also biologically active, making them suitable for site-specific conjugation to nanoparticles for imaging atherosclerotic plaques, where inflammation and immune responses contribute to plaque instability.
2. Phage Display Single-Domain Antibody Library for Antibody Development
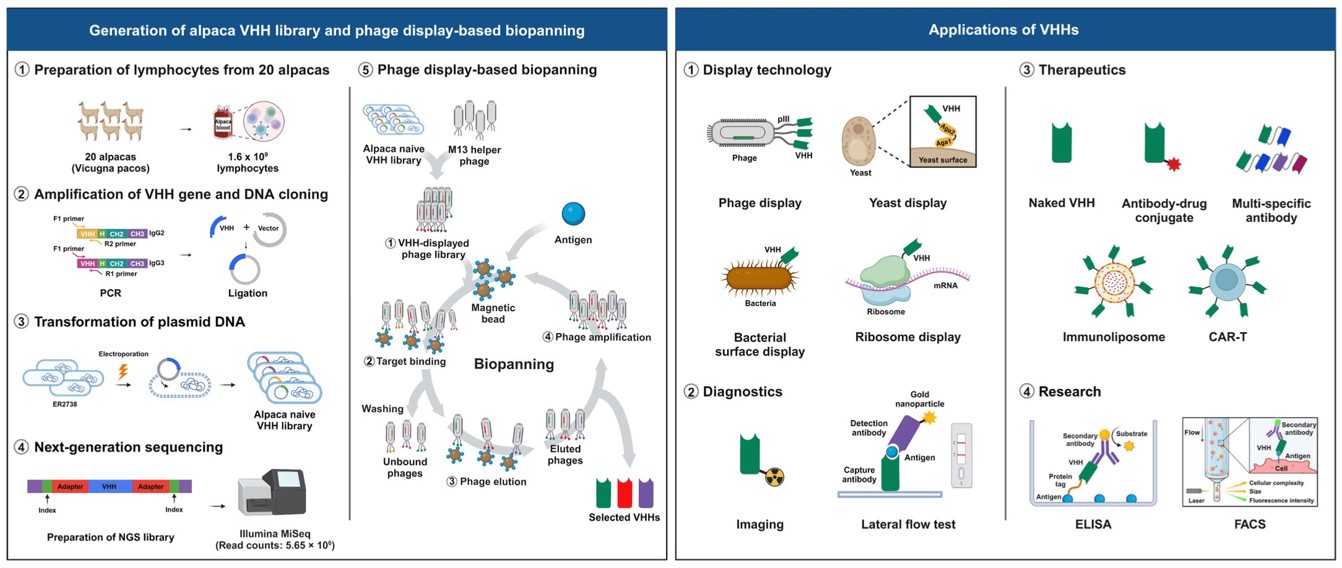 Fig.4 Construction of an alpaca VHH phage display library.4,5
Fig.4 Construction of an alpaca VHH phage display library.4,5
In this study, researchers constructed a large sdAb (VHH) phage display library using 1.6 × 109 lymphocytes from 1 L of blood collected from 20 non-immunized alpacas. Next-generation sequencing analysis confirmed the library's diversity, supporting its potential for antibody discovery. Systematic selecting of the library isolated multiple sdAbs with high affinity targeting three therapeutically relevant antigens. Overall, the alpaca sdAb phage display library represents a valuable resource for diagnostics and therapeutics, offering insights into the development of humanized synthetic sdAb libraries and holding promise for use in various antibody-based therapeutic platforms.
References
For Research Use Only.
