Creative Biolabs is a technology-based service provider specialized in the development of high-quality antibodies and in vitro diagnostic (IVD) immunoassays for global clients. Our hands-on expertise and years of experience producing antibodies allow us to provide our clients with the best-quality antibody (pairs). We provide custom production and validation services of antibodies against a wide spectrum of disease biomarkers. Areas of research include but are not limited to cancer, apoptosis, neurobiology, immunology, cardiovascular diseases, and infectious diseases.
For immunoassay development, our IVD immunoassay kit development expertise lies in different formats, including lateral flow, chemiluminescence assays, ELISA tests, turbidimetric assays, and rapid tests. Our services can be customized to fit different applications, to develop robust and specific assays/tests, and to make Creative Biolabs your partner of choice for assay development and manufacturing.
Creative Biolabs follows a phase-gated and regulated process for assay development. We offer one-stop custom-specific services to develop complete immunoassays on demand or offer flexible support in the different phases of the project. More practically, we offer support in assay design, raw material selection, and working protocol establishment. Moreover, we offer systematic evaluation and optimization of the variables of the assay to reach optimal performance. You remain in control of the project as at the end of each phase, a design review will be held to ensure that the project milestones are still valid and are being achieved.
Whether you need a one-time product or service, a combination of products and services, or a fully customized antibody/kit development program, we are ready to put our expertise and technologies to work for you. Antibody development or immunoassay development for both research and diagnostic use.
Specialized in the production of high-quality antibodies and IVDs that detect a broad range of markers, including hormone, tumor markers, food safety, infectious organisms, etc. Abundant expertise with know-how in all steps of the research, development, manufacture, and assembly process.
Quick response to your needs from the first step to the last by offering individualized technical support to our valued customers by learning and understanding their objectives and doing our best to accommodate them accordingly.
Cutting edge technologies, keen project, and program management skills, flexibility, and a client-focused approach enable us to provide in-depth expertise to tackle the obstacles challenging the clients' business.
Creative Biolabs has successfully developed a wide variety of immunoassays targeting different disease biomarkers. Bringing in years of experience and expertise, our dedicated team of professionals will help you with every step of the development process, thereby accelerating and bringing your immunoassay to market rapidly. Contact us to discuss your project and experience the great value of our services.
1. Development of a Novel Immunoassay for Presepsin (sCD14-ST) Detection
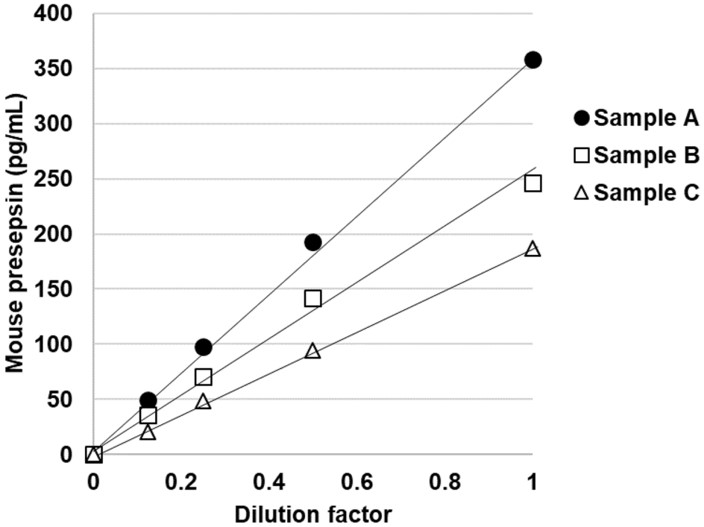 Fig.1 Linearity of the developed ELISA for mouse presepsin.1,3
Fig.1 Linearity of the developed ELISA for mouse presepsin.1,3
This study presented a sandwich enzyme-linked immunosorbent assay (ELISA) with high sensitivity for detecting presepsin in mouse plasma, designed to explore its association with diseases. Polyclonal antibodies were generated from rabbit antiserum immunized with peptides, and recombinant mouse presepsin-Fc fusion protein was expressed and purified for use as a standard. The method’s linear detection range was 4.7–300 pg/mL, with a detection limit of 1.4 pg/mL. The assay specifically detected mouse presepsin without cross-reactivity from soluble CD14 (sCD14), even after digestion by cathepsin D proteinase. This method provides high specificity and is valuable for investigating presepsin-related diseases.
2. Development of Lateral Flow Immunoassay with a Stacking Pad Design
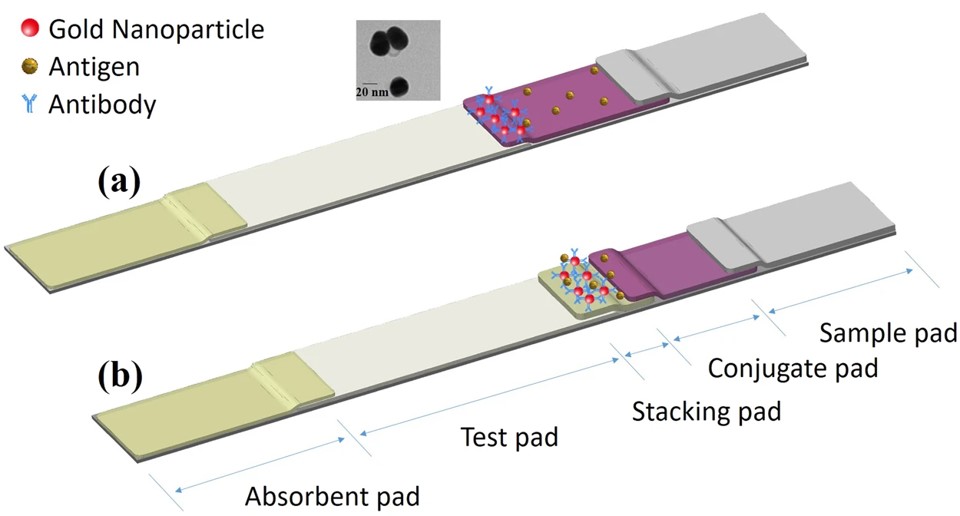 Fig.2 Proposed mechanism of the LFIA and sLFIA tests.2,3
Fig.2 Proposed mechanism of the LFIA and sLFIA tests.2,3
In this study, researchers developed an enhanced-sensitivity lateral flow immunoassay (sLFIA) without requiring additional steps or complex signal amplification. The method used a “stacking pad” configuration, which added an extra membrane between the conjugation pad and test pad in the conventional AuNP-based LFIA format. This design accumulates antibodies and antigens on the stacking pad, enhancing antigen/antibody binding interactions and improving detection sensitivity. The assay can detect as low as 1 ng/mL of Protein A and 15.5 ng/mL of C-reactive protein with the naked eye. It was successfully applied to analyze C-reactive protein in human serum and synovial fluid, offering a sensitive, on-site diagnostic tool for resource-limited settings.
References
For Research Use Only.
