Skin absorption is a route of administration for medication by which drugs can enter the body through the outer surface of the skin and treat skin-related disease. Assessing skin absorption level is contributed to improving the effect of drugs and reducing skin irritation, corrosion and sensitization. Creative Biolabs provides skin absorption measurement service to support your ADME program.
Dermal penetration occurs mainly by passive diffusion. Several factors can affect the rate of permeation of a substance through the skin, such as drug concentration, exposure period, product formulation, part of the body exposed, and dosage. Knowing the properties of the API has great help in understanding skin absorption. Creative Biolabs provides physicochemical characterization service to assess several API properties including octanol/water partition coefficient (logP), molecular size, ionization, surfactants, etc. to add dermal absorption studies. To provide more accurate data, Creative Biolabs keeps a good correlation between different skin diffusion measurements with the same chemical.
We have undertaken painstaking efforts to optimize our skills and expertise. Our service is fully in compliance with the in vitro dermal absorption assay of EFSA Journal 2012 Guidance, OECD Guidance Document No. 28, OECD Guidance Notes on Dermal Absorption, OECD Guideline Test No. 428 and OECD Guideline Test No. 427. We use split-thickness human skin or animal skin as in vitro models for static diffusion and flow-through diffusion for dermal penetration testing.
Static Diffusion Cell System
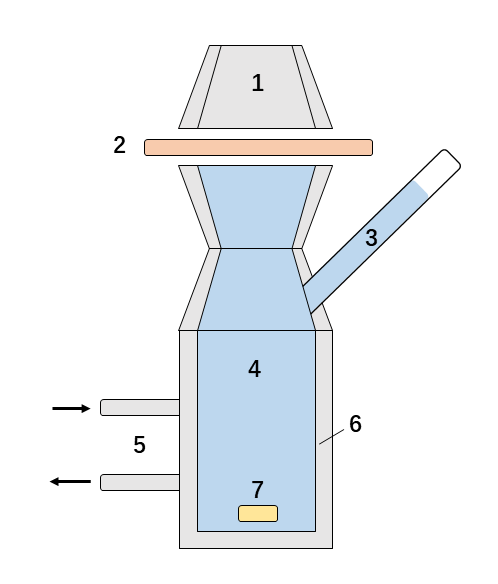 Fig. 1 Static diffusion cell.
Fig. 1 Static diffusion cell.
1. Donor chamber 2. Skin sample 3. Sampling port 4. Receptor chamber
5. Manifold filling the water jacket 6. Water (flow) jacket 7. Magnetic stirrer
The two main parts of static diffusion cell (Franz cell) system are a receptor and a donor chamber (Figure 1). The skin is inserted between these two compartments. The donor chamber and the receptor chamber are filled with receptor fluid (e.g. saline, ethanolic solution) and tested agent of the same dose is applied to the donor chamber. Exposure mimics a working day (e.g. 6-10 hours) with sampling for up to 24 hours. During the exposure period, the tested agent permeates through the skin and accumulates in the receptor chamber. This study can be studied using carbon/hydrogen labeled compounds (14C/3H) or using non-labeled compounds with HPLC-MS/MS-based analysis. The final result is derived from at least 4 repeats.
Flow-Through Diffusion Cell System
The in vitro flow-through diffusion system can achieve a more realistic drug absorption behavior. We use the automated in-line flow-through diffusion cells with both human and animal skins. We can provide both radiolabeled and unlabeled compounds, several layers of tape stripping. HPLC-MS/MS can be used for analysis.
Skin Samples
Creative Biolabs offers several skin samples, which covers rat skin, pig skin and human skin. Human skin is considered to be less permeable than that of laboratory animals. Our in vitro studies with human skin use split thickness (200-400/500 μm, dermatomed) skin as split thickness membranes tend to have lower levels of residual material than full-thickness preparations. The split thickness skin can be derived from the abdomen, back, breast or upper leg according to your need.
Creative Biolabs can design tailored-made studies to demonstrate the absorption efficiency of a product in a specific layer of the skin. We also provide in vivo services to support skin irritation, corrosion and sensitization risk evaluation of drug candidates. For more detailed information, please feel free to contact us or directly sent us an inquiry.
For Research Use Only.


 Fig. 1 Static diffusion cell.
Fig. 1 Static diffusion cell.

