Glycosylated antibodies are extremely important to a variety of biological processes but their analysis is a big challenge because of the low abundance and heterogeneity of glycans. Glycan analysis can profile the glycan structure and monitor the relative quantities of a particular set of glycans. As a forward-looking company as well as a market leader in the field of therapeutic antibody development, Creative Biolabs has successfully developed a versatile antibody development and analysis platform to illustrate glycan structure and relative quantities of antibody and screen the changes in glycosylation. We are glad to work with you in your antibody glycan analysis to help you get milestone success.
Glycan Analysis is Needed for Antibody Engineering
Glycosylation is an important attribute of biopharmaceutical products [such as monoclonal antibodies (mAbs) and therapeutic glycoproteins] to monitor from development through production to ensure proper function, efficacy, and safety, stability, serum half-life, etc. For therapeutic mAb engineering, glycan analysis can profile the glycan structure and monitor the relative quantities of a particular set of glycans, which is extremely important due to the important role of glycosylation in determining antibody characteristics and functions. Glycan analysis can also provide valuable information regarding changes in glycan patterns or identify aberrant glycans, which may have adverse effects on human physiological conditions and accompany many chronic and infectious diseases. As a result, glycan analysis has been routinely performed in mAb glycoengineering.
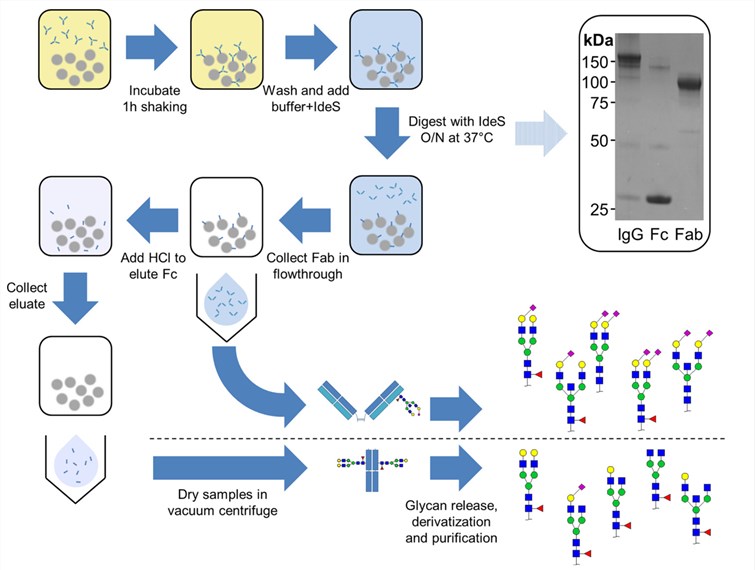 Fig.1 Workflow of IgG Fab and Fc glycosylation analysis. (Bondt, 2014)
Fig.1 Workflow of IgG Fab and Fc glycosylation analysis. (Bondt, 2014)
N-Glycan Analysis
N-linked oligosaccharide is covalently bonded with the nitrogen of asparagine residue and all N-linked proteins share a basic core structure of conserved pentasaccharide (GlcNAc2Man3) backbone. For immunoglobulins, N-linked oligosaccharides have received particular attention because changes in the attached glycans can impact immunoglobulin solubility, structural stability, and biological functions. For example, the differential of single monosaccharide at Asn-297 glyco-site at the Fc fragment of human IgG can drastically affect IgG binding to FcγR and affecting complement action. Technologies for N-Glycan analysis provided by Creative Biolabs including but not limited to:
-
HPAEC-PAD
High-performance anion exchange chromatography coupled with pulsed amperometric detection (HPAEC-PAD) is a selective and specific glycan analysis method that separates carbohydrates with specific interactions between the hydroxyl and carboxyl groups of glycans. Generally, samples are acid hydrolyzed by peptide-N-glucosidase F (PNGaseF) to release the N-linked glycans and then analyzed by HPAEC-PAD. It has been as a powerful tool to analyze the total glycosylation of a protein, the types of glycans, the amounts of specific monosaccharides and the carbohydrate sequence of a glycan. Moreover, HPAEC-PAD can detect the aberrant differences in glycan patterns that may indicate disease conditions. The qualitative and quantitative estimation values are standardized by known N-linked glycans.
-
HPLC
High-performance liquid chromatograph (HPLC) is also a common method for the characterization of antibody glycan. HPLC is usually used conjugated with different detection methods, such as fluorescence-based detectors (HPLC-FD) or mass spectrometers (HPLC-MS). Before HPLC-FD analysis, glycans must be labeled with some fluorescent tags [usually 2-aminobenzamide (2-AB) or 2-aminobenzoic acid (2-AA)]. Highly efficient hydrophilic interaction chromatography (HILIC) is the most widely used chemistries column for glycan analysis. The qualitative and quantitative values are standardized by known N-linked glycans.
-
Mass Spectrometry (MS)
Mass spectrometry is a versatile technique that has increasingly been used in structural elucidation of glycans due to its sensitivity, potential for high throughput, and the capability for tolerating diverse biological mixtures. Separation techniques such as liquid chromatography (LC), ion mobility are typically used in conjunction with mass spectrometry to analyze the antibody monosaccharide with more accuracy.
O-Glycan Analysis
O-acetylgalactosamine (O-GalNAc) and O-acetylglucosamine (O-GlcNAc) are the common O-glycosylations types, among which, O-GalNAc is attached to the hydroxyl group of the protein serine or threonine residues through an α-linkage, while O-GlcNAc is attached through a β-linkage. Technologies for O-Glycan analysis provided by Creative Biolabs including but not limited to:
-
MALDI-TOF MS
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is a high-throughput method to analyze the structural characterization of glycosylated compounds with high sensitivity and robustness. Before O-glycans profiling, permethylation of the O-glycan to transform all hydroxyl groups into methyl ethers and stabilizes sialic acids by methyl esterification of their carboxyl groups is necessary, which allows MALDI-TOF MS to analyze of all O-glycans in the positive mode.
-
HPAEC-PAD
HPAEC-PAD can also provide the characterization of O-linked glycans, but unlike N-Glycan analysis, O-linked glycans can be cleaved from the proteins using alkaline sodium borohydride (NaBH4) in a β-elimination reaction. Followed by the release from the proteins, O-linked glycans can be analyzed to illustrate the total glycosylation, the types of glycans, the amounts of specific monosaccharides and the carbohydrate sequence of a glycan.
Why Choose Us?
-
High throughput with sensitivity and specificity
-
Stability and consistency, without a large scale of repeated trials
-
One-stop service with fully customized design
-
Competitive prices and best after-sale service
Characterizing the antibody glycans including glycan macroheterogeneity (glycosylation site occupancy) and microheterogeneity (site-specific glycan structure) is important for the understanding of glycosylated antibody biosynthesis and function. With years of experience and an experienced scientific team, Creative Biolabs has developed, optimized, and validated a panel of platforms to offer high-quality antibody glycan analysis services. Additionally, we can offer a fully customized project design to satisfy any specific requirement. If you are interested, please contact us or directly sent us an inquiry.
Reference
-
Bondt, Albert, et al. "Immunoglobulin G (IgG) Fab glycosylation analysis using a new mass spectrometric high-throughput profiling method reveals pregnancy-associated changes." Molecular & Cellular Proteomics 13.11 (2014): 3029-3039.
Distributed under Open Access License CC BY 4.0, without modification.
For Research Use Only.


 Fig.1 Workflow of IgG Fab and Fc glycosylation analysis. (Bondt, 2014)
Fig.1 Workflow of IgG Fab and Fc glycosylation analysis. (Bondt, 2014)


