Over the past decade, Creative Biolabs has supported a diverse range of antibody function assay studies and provides receptor occupancy (RO) assay services to facilitate your project success.
What is RO assay
Receptor occupancy (RO) assays are commonly used to quantify the binding of therapeutic drugs to their cell surface targets. The RO data can provide some reference for pharmacodynamic (PD) biomarker data; meanwhile, it can establish PK/PD relationships when combined with the pharmacokinetic (PK) profile.
Commonly, receptor occupancy (RO) assays are measured by flow cytometry. As quantitative assays, flow cytometry-based RO assays could evaluate both the receptor binding and other pharmacodynamic characteristics of experimental therapies. Under different research scenarios, receptor occupancy (RO) assays can be designed to be relatively simple to measure the number of receptors for the binding drug, as well as more complex experiments to evaluate the internalization of receptors after the binding drug.
RO Assay Formats
These are three basic formats that have been used as PD measurements in drug development.
 Fig.1 Three different formats of Receptor Occupancy (RO) assays.1
Fig.1 Three different formats of Receptor Occupancy (RO) assays.1
Free Receptor Assay
The first format is a critical measurement when evaluating the cell specificity and therapeutic dose of an antibody. In this format measurement, the proportion of unbound or unoccupied receptors by using a fluorescence-labeled detection reagent to the receptor.
Drug-occupied Receptor Assay
For the second RO assay format measuring drug-occupied receptors, a fluorescence-labeled anti-drug antibody (ADA) is generally used for detection based on non-competing with drug binding, and the proportion of receptors bound by the drug is determined by the measurement of the bound drug.
Total Receptor Assay
The total receptor assay is commonly employed for biotherapeutic drugs with a mechanism of ADCC action. For the third format including both free and drug-occupied receptors, the detection reagent is usually an anti-receptor antibody that binds to an epitope distinct from that of the drug (i.e., a non-competing antibody).
RO Assay Services in Creative Biolabs
Creative Biolabs has developed state-of-the-art, GMP-compliant platforms that integrate the latest technologies and equipment for scientific research. Our team of Ph. Ph.D.-level scientists brings a wealth of expertise and experience to the table, ensuring meticulous custom assay design, rigorous reagent characterization, precise data normalization/reporting, and comprehensive implementation planning. This enables us to deliver reliable, high-quality RO assay results with a quick turnaround time, meeting the needs of our global customers.
Representative Data
This research report examines two independent flow cytometry techniques for determining CCR5 receptor occupancy (RO) using the anti-CCR5 antibody. Findings indicate that both methods yielded similar CCR5 RO values, exhibited minimal background in untreated CCR5+CD4+ T cells, and enabled sensitive occupancy assessments on both blood-derived and tissue-resident CD4+ T cells. These measurements demonstrated a longitudinal correlation with plasma concentrations in the anti-CCR5 antibody-treated macaques.
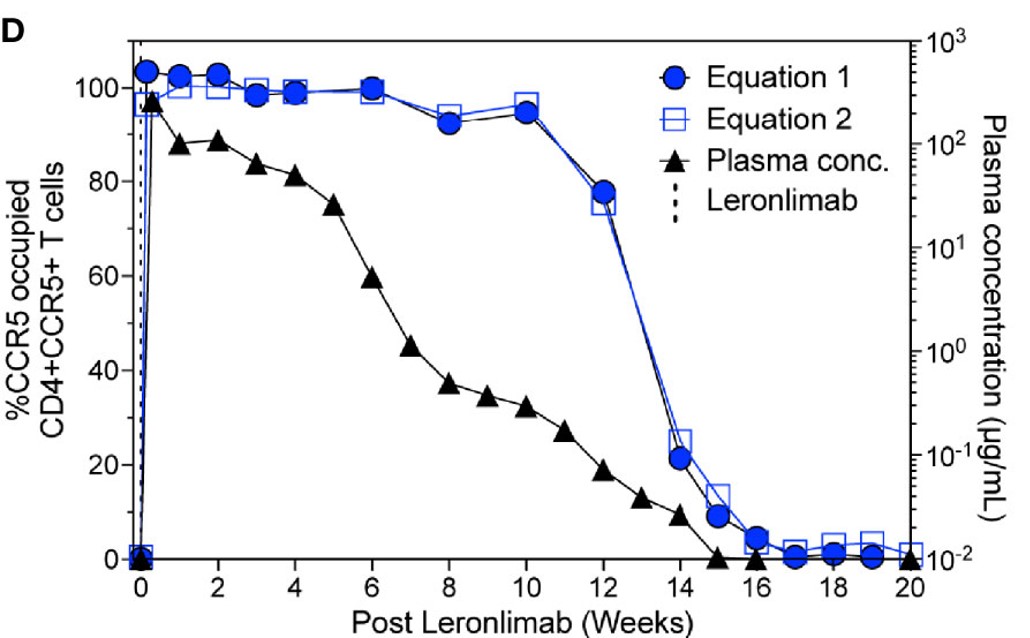 Fig.2 CCR5 receptor occupancy.2
Fig.2 CCR5 receptor occupancy.2
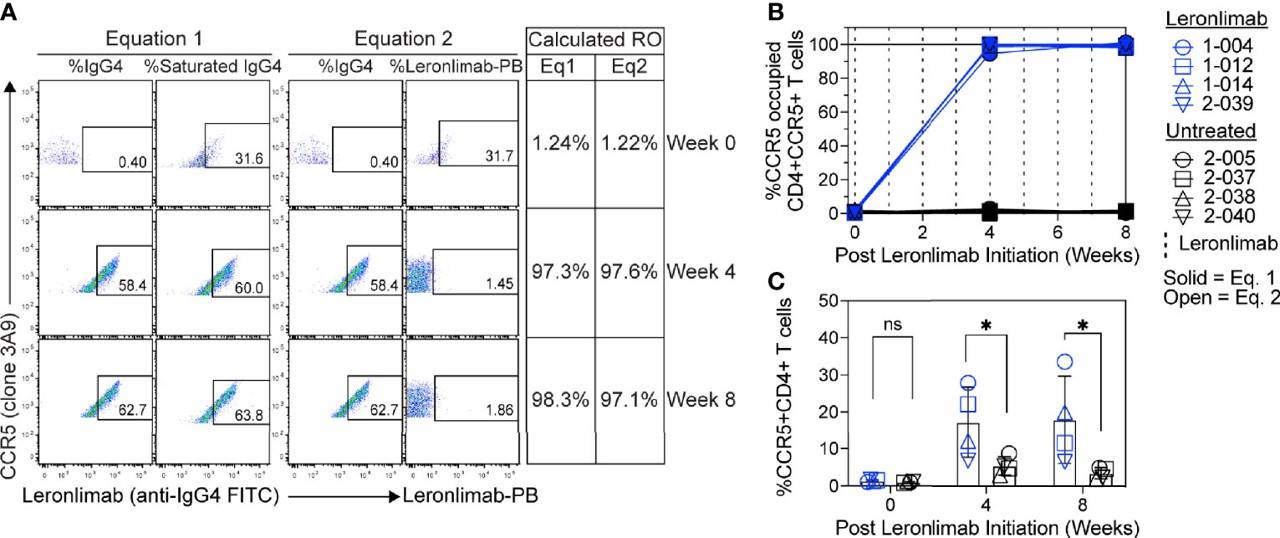 Fig.3 RO test for CCR5 in human samples.2
Fig.3 RO test for CCR5 in human samples.2
Features of Our Services
-
Extensive experience in assay development platform.
-
Advanced techniques and custom assay service.
-
Excellent quality and timely feedback.
If you are interested in our services, please contact us to discuss your project.
References
-
Aru, Basak, and Gulderen Yanikkaya Demirel. "Flow Cytometry: A Versatile and Powerful Tool for Drug Discovery and Development." Pharmedicine Journal 1.1 (2024): 1-19.
Distributed under Open Access License CC BY 4.0, without modification.
-
Chang, Xiao L., et al. "CCR5 receptor occupancy analysis reveals increased peripheral blood CCR5+ CD4+ T cells following treatment with the anti-CCR5 antibody leronlimab." Frontiers in Immunology 12 (2021): 794638.
Distributed under Open Access License CC BY 4.0. The original image Figure 2 was modified by extracting and using part D, and the title was changed to "CCR5 receptor occupancy". The original image Figure 5 was not modified.
For Research Use Only.


 Fig.1 Three different formats of Receptor Occupancy (RO) assays.1
Fig.1 Three different formats of Receptor Occupancy (RO) assays.1
 Fig.2 CCR5 receptor occupancy.2
Fig.2 CCR5 receptor occupancy.2
 Fig.3 RO test for CCR5 in human samples.2
Fig.3 RO test for CCR5 in human samples.2


