- Home
- Applications
- Breast Cancer
ADC Development Services for Breast Cancer Research
Despite great advances have been made in cancer research, breast cancer remains to be a major health problem and represents currently a top biomedical research priority. Integrating the tumor specificity provided by unique monoclonal antibody (mAb) and the cytotoxicity of small molecule drugs, antibody-drug conjugates (ADCs) are a series of smart therapeutics that have recently shown great promise in treating various cancers. Equipped with advanced technology platform and talented scientists, Creative Biolabs provides customized ADC development services against breast cancer for our worldwide clients to help their anti-breast cancer ADC development.
Introduction of Breast Cancer
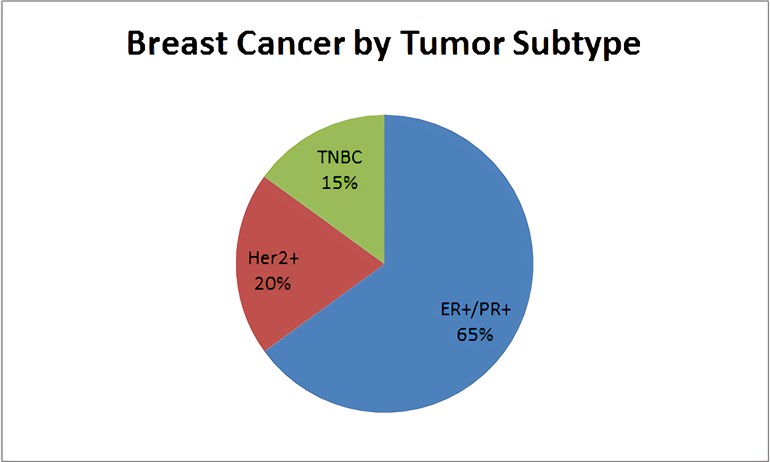
Breast cancer is a heterogeneous group of malignancies with a spectrum of molecular subtypes, pathologies and outcomes that together comprise the most common non-cutaneous cancer in women, with roughly half a million deaths per year worldwide. Breast cancers are classified through three protein expression markers: progesterone receptor (PgR), estrogen receptor (ER), and the overexpression of the growth factor receptor HER2. Hormonal therapies, including tamoxifen and aromatase inhibitors, can be effective in treating tumors that express the hormone receptors ER and PgR. HER2-directed therapies are useful for tumors that express HER2. Triple-negative breast cancer (TNBC), defined as the absence of ER, PgR, or HER2 expression, comprises 15% to 20% of breast cancers. TNBC has a high mortality rate and is more likely to recur than the other 2 subtypes because of its aggressive behavior, with a median survival of 10-13 months from time of metastasis.
Diagnosis and Treatment of Breast Cancer
Currently, various imaging techniques such as mammography, magnetic resonance imaging (MRI), positron-emission tomography (PET), computed tomography (CT), and single-photon emission computed tomography (SPECT) could be used for diagnosis and monitoring patients with breast cancer in various stages. Among various diagnosis platforms, imaging techniques are main diagnosis approaches which could provide valuable data on patients with breast cancer. Besides, utilization of biochemical biomarkers such as DNAs, mRNAs, microRNAs, and proteins, could be employed as new diagnosis and therapeutic tools for patients with breast cancer. Depending on clinical tumor subtype, therapeutic backbones include endocrine therapy, anti-HER2 targeting, and chemotherapy. Among various approaches for breast cancer treatment, chemotherapy is predominantly used for patients at stages II-IV. Monoclonal antibodies, ADCs, tyrosine kinase inhibitors, cyclin-dependent kinase inhibitors, mammalian target of rapamycin (mTOR) inhibitors, and PARP inhibitors are types of targeted therapies used in the treatment of breast cancer. However, some of the mentioned treatments may cause side effects that continue or appear months or years after treatment has ended, called late effects.
- Late effects of radiation therapy include:
- Inflammation of the lung after radiation therapy to the breast, especially when chemotherapy is given at the same time.
- Arm lymphedema, especially when radiation therapy is given after lymph node dissection.
- In women younger than 45 years who receive radiation therapy to the chest wall after mastectomy, there may be a higher risk of developing breast cancer in the other breast.
- Late effects of chemotherapy depend on the drugs used, but may include:
- Heart failure.
- Blood clots.
- Premature menopause.
- Second cancer, such as leukemia.
- Late effects of targeted therapy with trastuzumab, lapatinib, or pertuzumab may include heart problems such as heart failure.
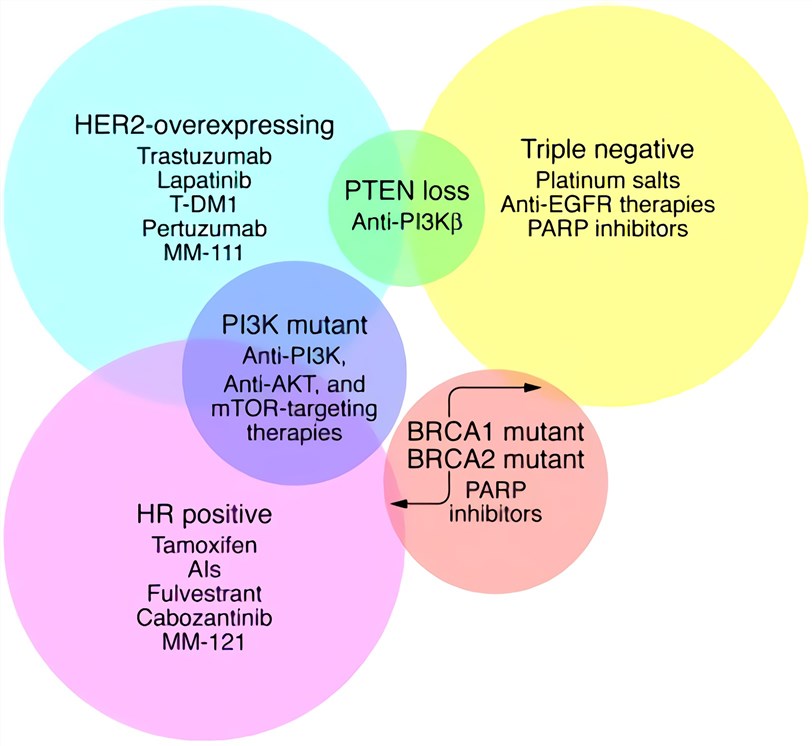 Fig.1 Venn diagram of breast cancer subtypes and their overlapping molecular targets. (Higgins, 2011)
Fig.1 Venn diagram of breast cancer subtypes and their overlapping molecular targets. (Higgins, 2011)
ADC Therapy for Breast Cancer
Targeted therapy using ADCs holds great promise for the treatment of breast cancer. ADC represents a promising strategy for patients with breast cancer especially metastatic TNBC, with early clinical trial results suggesting that novel agents could eventually change the landscape for this patient population. Ado-Trastuzumab emtansine (T-DM1) was the first and the only ADC approved by the US Food and Drug Administration (FDA) for HER2-positive breast cancer. Following the success of T-DM1, many novel anti-breast cancer ADCs have been developed, and their anticancer efficacies are currently undergoing preclinical or clinical investigation. For example, CDX-011, an anti-GPNMB antibody conjugated to monomethyl auristatin E, has shown early efficacy in TNBC in an ongoing phase II clinical trial. In addition, IMMU-132, BAY94-9343, SGN-LIV1A, and BAY1129980 are ADCs in the early stages of clinical development with potential in the treatment of breast cancer.

ADC Development Services
One approach to improve breast cancer therapy is designing drugs directed against targets with differential levels of expression on malignant versus normal cells with the goal of improving tumor selectivity and reducing damage to normal tissues, such as ADCs. Over the years, a number of biomarkers have been proven to be good targets for ADC development, including CA6 ADC Service, GPNMB ADC Service,TROP2… As a leader in the ADC development field, Creative Biolabs offers one-stop ADC development services during the whole process. Our services include ADC Antibody Screening, Antibody Design and Conjugation, DrugLnk™ Custom Synthesis, ADC in Vitro Analysis, ADC in vivo Analysis…
ADCs show great potential for developing targeted therapies against breast cancer, as well as other malignancies. Creative Biolabs has effectively combined a comprehensive range of conjugation and analysis technology to support the full spectrum of activities involved in biologic ADC drug development. If you are interested in our ADC development services for breast cancer, please feel free to contact us for more information.
Reference
- Higgins, M. J.; Baselga, J. Targeted therapies for breast cancer. The Journal of clinical investigation. 2011, 121(10): 3797-3803.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
