- Home
- Services
- Fragment-drug Conjugates Development
- Antibody Fragment-drug Conjugates Development
Antibody Fragment-drug Conjugates Development
Antibody fragment-drug conjugates (FDCs) combine the pharmacological potency of highly cytotoxic drugs with the high specificity of an antibody fragment against tumor-associated targets. Similar to antibody-drug conjugates (ADCs), FDCs consist of three major components, and the biggest difference is the targeting component. FDCs comprise much smaller antibody fragments such as antigen binding fragment (Fab), single-chain variable fragment (scFV), small immuno-proteins (SIPs), diabody, sdAb. As a senior ADC supplier, Creative Biolabs now fully focuses on the much wider market opportunity of FDCs and has applied its technology and expertise using conventional cytotoxic payloads, whilst retaining its knowledge and capabilities in antibody fragments expression as well as engineering, synthetic chemistry and conjugation science. We now provide customized antibody fragment based-FDC development services for our honor clients.
Antibody Fragment-drug Conjugates
When considering smaller formats of ADCs, antibody fragments are an obvious choice. The pre-clinical and clinical pipeline of antibody fragments continues to expand with a growing number under evaluation as FDCs. For all therapeutic indications, the hope is that antibody fragments will offer differentiated performance compared to whole immunoglobulin (Ig) G antibodies, primarily due to altered pharmacokinetics (PK) and tissue distribution as a consequence of their smaller size. For FDCs, there is the potential for faster penetration into solid tumors but also for faster clearance from systemic circulation. High tumor-to-blood ratios and reduced systemic toxicity will be achieved by using FDCs to enhance the therapeutic index. Additionally, as antibody fragments lack an Fc domain, there is an absence of antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC).
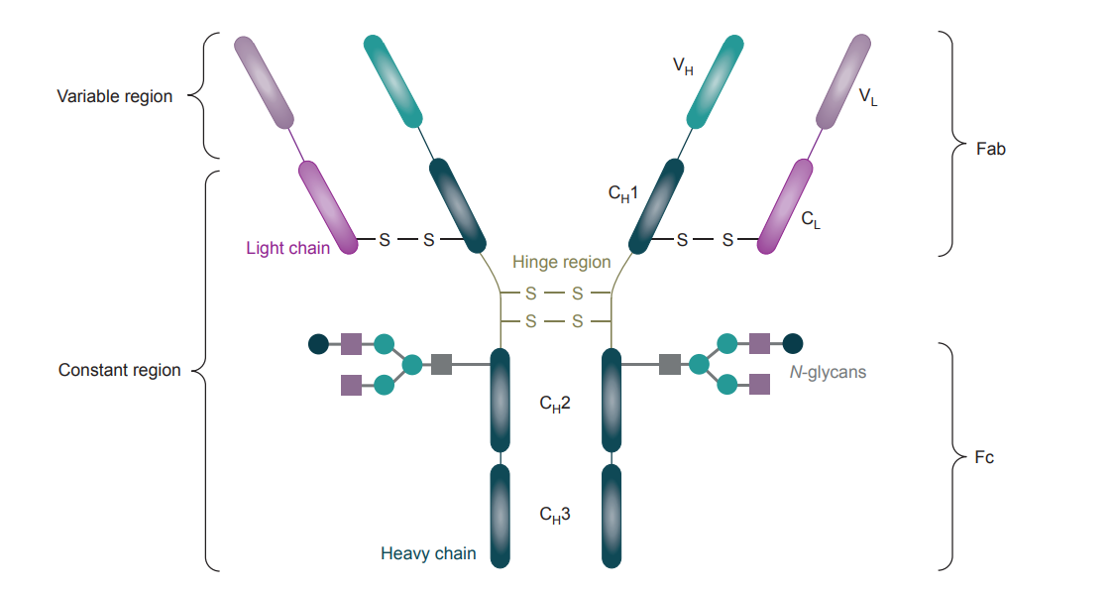 Fig.1 General structure of an IgG1 highlighting key components. (Chudasama, 20016)
Fig.1 General structure of an IgG1 highlighting key components. (Chudasama, 20016)
Fab-drug Conjugates
Fab fragments comprise the constant and variable domains of Ig, linked by a single disulfide bond present at the C-terminus. They are arguably the most explored fragment format in the clinic and ideal for drug conjugation via bis-alkylation conjugation as they possess a single, solvent accessible inter-chain disulphide bond. Studies have shown that conjugation at the inter-chain disulphide of a Fab is highly selective, leading to homogeneous conjugates and ideally situated distal to the binding domain. Thus, conjugation would be expected to have a minimal impact on the binding properties of the Fab. Engineered approaches involving the introduction of a cysteine residue at a specific location are also emerging.
ScFv-drug Conjugates
ScFv is comprised of the heavy and light chains of the variable region of an Ig linked by a short amino acid spacer, disulfide bond, or both. The small size of scFv makes it suitable for expression in bacterial and yeast systems. It also makes them ideal candidates for high-throughput selection technologies such as cell display, phage display, yeast display, and ribosomal display. This allows large libraries of clones, mutated in key binding sites, to be screened against a target. Through multiple rounds of selection, high-binding clones against disease targets can be identified and expressed. Since scFv fragments are normally produced via expression, the process of site-selective payload conjugation is simplified. Reactive amino acids can easily be introduced to specific locations on the fragment using site-directed mutagenesis and subsequently exploited for highly-controlled payload conjugation.
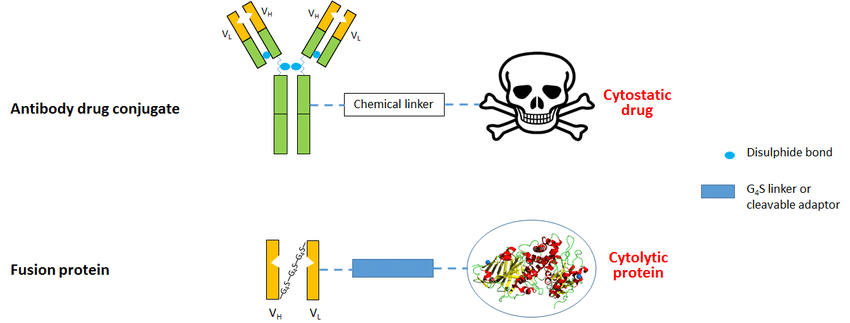 Fig.2 Immunotoxins comprising IgG or scFv linked to an anti-cancer agent. (Jordaan, 2017)
Fig.2 Immunotoxins comprising IgG or scFv linked to an anti-cancer agent. (Jordaan, 2017)
Small immunoprotein-drug Conjugates
Small immunoproteins (SIPs) comprise scFv fragments fused to an Ig-derived constant region. The presence of these constant regions facilitates non-covalent dimerization of two monomers, producing bivalent binders. Chemical conjugation to SIPs is commonly achieved via site-specific incorporation of a cysteine residue into the constant domain of the monomer. Since SIPs are commonly expressed in mammalian cells, features such as constant domain, linker length, and the presence of site-specific amino acids can be easily altered.
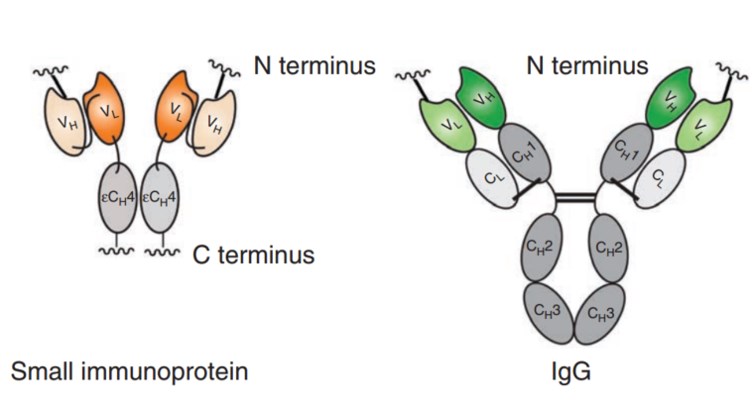 Fig.3 Comparison of SIP format and whole IgG. (Bernardes, 2013)
Fig.3 Comparison of SIP format and whole IgG. (Bernardes, 2013)
sdAb-drug Conjugates
A VHH antibody, also known as sdAb, single domain antibody (sdAb), is the antigen binding fragment of a heavy chain only antibodies. VHH antibodies are considered the smallest naturally derived Fab that can be isolated from a full-sized Ig. The unprecedented biophysical properties and small size of the VHH domains make them ideal building blocks for developing novel, differentiated domain antibody-drug therapeutics.
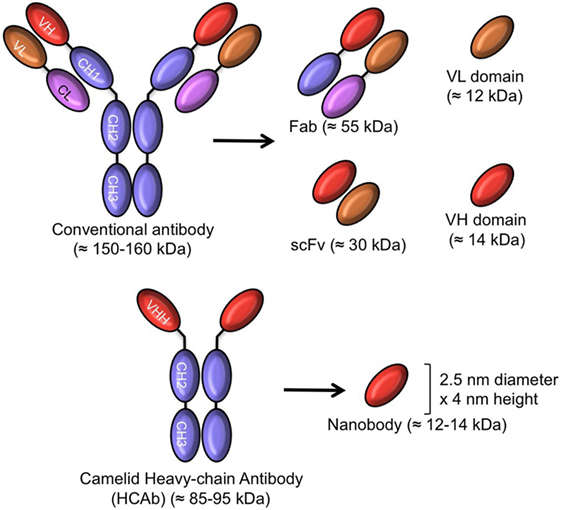 Fig.4 Schematic representation of intact antibody and antibody-derived fragments. (Arezumand, 2017)
Fig.4 Schematic representation of intact antibody and antibody-derived fragments. (Arezumand, 2017)
Advantages over Intact IgG Antibody
- Faster penetration into solid tumors
- Faster clearance from systemic circulation
- High tumor-to-blood ratios
- Reduced systemic toxicity
- Enhanced therapeutic index
- Easy to discover and can be bioengineered for multiple drug-molecule conjugations
Our novel approach uses small antibody fragments, which incorporate our revolutionary protein expression systems and DrugLnk technology platform, to yield FDC products with improved characteristics, such as high drug to antibody ratio (DAR). These high DAR FDCs have been shown to penetrate tumors more rapidly than conventional ADCs, enabling them to act faster and clear more rapidly from normal tissues. This reduces side-effects, achieves a wider therapeutic index, and delivers a significantly superior therapeutic benefit. In combination with in-house engineering expertise, Creative Biolabs can efficiently identify and provide optimal fragment formats for engaging therapeutically valuable targets. If you are interested in our antibody fragment based FDC development services, please don’t hesitate to contact us for more information.
References
- Chudasama, V.; et al. Recent advances in the construction of antibody-drug conjugates. Nature chemistry. 2016, 8(2): 114-119.
- Jordaan, S.; et al. CSPG4: a target for selective delivery of human cytolytic fusion proteins and TRAIL. Biomedicines. 2017, 5(3): 37.
- Bernardes, G. J. L.; et al. Site-specific chemical modification of antibody fragments using traceless cleavable linkers. Nature protocols. 2013, 8(11): 2079-2089.
- Arezumand, R.; et al. sdAbs as novel agents for targeting angiogenesis in solid cancers. Frontiers in immunology. 2017, 8: 1746.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
