- Home
- UTC Development
- Bispecific ADC Development
- Biparatopic Receptor based Bispecific ADC Development
Biparatopic Receptor based Bispecific ADC Development Services
Antibody-drug conjugate (ADC) represents an innovative and potent therapeutic biopharmaceutical drug. It combines the unique and sensitive targeting capabilities of antibodies allowing sensitive discrimination between healthy and cancer tissues with the cell-killing ability of cytotoxic drugs. To date, eight ADCs have been approved and more than 100 ADCs are currently in pre-clinical and clinical trials.
The key challenge during the development of a successful ADC is balancing its efficacy and safety. Bispecific antibodies presenting the ability to simultaneously engage two targets are endowing some creative possibilities to improve both the efficacy and safety of ADCs. Currently, several strategies have been developed using bispecific antibodies to enhance ADC internalization and lysosomal delivery, thereby to enhance the efficacy of ADCs, such as the bispecific and biparatopic ADCs. Creative Biolabs is a leader in the field of providing ADCs development services. We now provide a fully integrated biparatopic receptor-based bispecific ADCs design and construction services against different markers for the treatment of different cancers.
Background
Bispecific Biparatopic Antibody
Bispecific antibodies (bsAbs) are engineered antibodies designed to recognize two different epitopes or antigens. BsAbs combine specificities of two antibodies against different antigens or epitopes that can interfere with multiple surface receptors or ligands. BsAbs offer the potential to maximize the benefits of therapeutic antibodies by various mechanisms, including simultaneously blocking two different targets or mediators that have a primary role in the disease pathogenesis, retargeting to mediate effector functions (such as antibody-dependent cell-mediated cytotoxicity (ADCC)), avoiding or delaying the development of resistance, and activating cytotoxic T and NK cells to induce tumor lysis. So far, there are more than 100 different bispecific formats endowing researchers to select the optimal parameters (such as size, half-life, stability, flexibility, orientation, and developability) to achieve the desired therapeutic outcome.
Biparatopic antibody is a subset of bispecific antibodies in which each antigen binding domain recognizes unique, non-overlapping epitopes on the same target antigen. Biparatopic antibodies have been confirmed that possess the superior ability to promote receptor clustering for improved receptor internalization, lysosomal trafficking, and receptor down regulation, therefore increasing drug potency.
 Fig.1 Various bispecific antibodies (bsAbs). 1
Fig.1 Various bispecific antibodies (bsAbs). 1
Bispecific Biparatopic ADCs
Recently, bispecific ADC approaches have emerged to enhance internalization and trafficking to the lysosome, thus maximizing the amount of drug that is effectively delivered to tumor cells at a given dose. Previous research suggested that targeting a single receptor with bispecific antibodies that recognize distinct epitopes could lead to increased avidity/overall affinity toward the target and greater potency. Subsequent studies confirmed that non-overlapping antibody pairs and biparatopic antibodies or non-antibody scaffolds could drive receptor clustering and cross-linking, which can enhance the internalization, trafficking to the lysosome, and degradation of the target.
By combining two non-overlapping HER2-targeted antibodies, researchers recently developed a bivalent biparatopic ADC in which the antibody has two engineered cysteines in the Fc region to enable site-specific functionalization conjugated to a potent tubulysin warhead. The ADC showed enhanced HER2 clustering and lysosomal trafficking, and potent target-mediated cytotoxic activity. Besides, the biparatopic ADC had more potent target-mediated cytotoxic activity in cancer cell lines and tumor models compared with monospecific ADC T-DM1. The biparatopic ADC also presents positive anti-tumor activity in tumor models with acquired resistance to T-DM1. Similarly, ZW49, another novel anti-HER2 biparatopic ADC that targets the same domains as trastuzumab (ECD4) and pertuzumab (ECD2) was developed. ZW49 is composed of a biparatopic antibody linked to a novel Nacyl sulphonamide auristatin payload via a protease cleavable linker. Preclinical study revealed that ZW49 can be internalized and trafficked to lysosomes in HER2-expressing cells to greater levels and faster than monospecific ADC (such as the TDM1). It also presented superior tumor growth inhibition relative to other HER2 targeted therapies in low HER2 patient-derived xenograft model and a trastuzumab-resistant high HER2 model. To date, ZW49 was selected to move forward into a phase I study.
Other biparatopic antibodies targeting different targets are also developed, which can be potentially used for biparatopic bispecific ADC development.
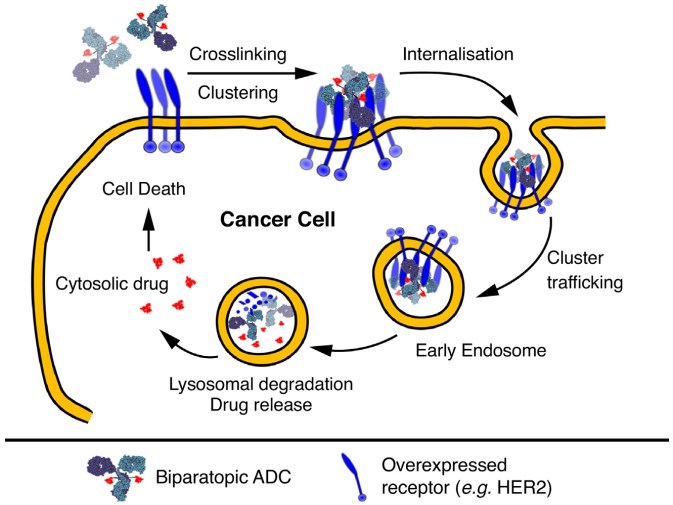 Fig.2 Schematic mechanism of action of biparatopic ADCs relying on crosslinking and clustering of receptors to enhance endocytosis and trafficking to the lysosome to deliver their payload.2
Fig.2 Schematic mechanism of action of biparatopic ADCs relying on crosslinking and clustering of receptors to enhance endocytosis and trafficking to the lysosome to deliver their payload.2
Our Service
Bispecific Biparatopic ADCs Development Services
Scientists at Creative Biolabs have enriched experience in antibody development and antibody/protein-drug conjugation for more than ten years. At present, we provide a full range of biparatopic receptor-based bspecific ADCs design and development services against a variety of targets. Our best-quality services include specific antibody development, potent drug or linker-payload production, and effective bio-conjugation with unbeatable rapid turnaround times. Our biparatopic ADCs preparation services target different targets including but not limited to:
Highlights
Bispecific Biparatopic ADCs Development Services
- Customized Antibody Engineering: Creative Biolabs likely offers tailored engineering of bispecific antibodies to target specific epitopes on cancer cells, enhancing the specificity and efficacy of the ADCs.
- High-efficiency Conjugation Technologies: They probably use advanced conjugation techniques to link the drug payload to the antibodies efficiently, ensuring stability and functionality of the ADCs.
- Comprehensive Analytical Services: Comprehensive in vitro and in vivo testing services to evaluate the efficacy, toxicity, and pharmacokinetics of the ADCs, ensuring they meet the required standards for further development.
- Innovative Drug-Linker Synthesis: Development of custom linkers that optimize the release of the drug payload at the target site, improving the therapeutic index of the ADCs.
FAQ
-
Q: How do biparatopic receptor-based bispecific ADCs improve therapeutic efficacy?
A: By targeting two different epitopes, biparatopic receptor-based bispecific ADCs can bind more tightly and specifically to cancer cells, improving drug delivery directly to the tumor. This dual engagement can lead to more efficient internalization and enhanced cytotoxic effects, ultimately improving the overall therapeutic outcome.
-
Q: What are the potential benefits of using biparatopic receptor-based bispecific ADCs over traditional ADCs?
A: The main benefits include increased specificity and binding affinity to the target cells, reduced off-target toxicity, and potentially overcoming resistance mechanisms that single-target ADCs might encounter. This approach can lead to improved safety and efficacy profiles in clinical applications.
-
Q: What are some challenges in developing biparatopic receptor-based bispecific ADCs?
A: Challenges include the complexity of designing and manufacturing bispecific antibodies, ensuring the stability and correct folding of the bispecific molecules, and optimizing the linker and drug payload to achieve the desired therapeutic effect. Additionally, ensuring the selective targeting of cancer cells without affecting healthy tissues remains a critical challenge.
-
Q: Can biparatopic receptor-based bispecific ADCs be used to target solid tumors?
A: Yes, biparatopic receptor-based bispecific ADCs have the potential to target solid tumors effectively. The dual-targeting approach can enhance tumor penetration and retention, potentially overcoming some of the limitations faced by traditional ADCs in treating solid tumors. Research and clinical trials are ongoing to explore and validate their efficacy in various solid tumor types.
-
Q: What custom services does Creative Biolabs offer for biparatopic ADC development?
A: Creative Biolabs offers custom antibody design and conjugation services, tailored to specific research needs, including antibody screening, DrugLnk™ custom synthesis, in vitro and in vivo analysis, and optimization of linker-payload combinations.
Published Data
The article discusses the development and evaluation of a biparatopic antibody-drug conjugate (ADC) targeting HER2, aimed at improving cancer treatment efficacy. This biparatopic ADC combines two different antibodies to enhance HER2 clustering and internalization, leading to efficient lysosomal degradation of the receptor-antibody complex. This approach aims to increase the cytoplasmic delivery of the cytotoxic agent, overcoming the limitations of current HER2-targeted therapies like T-DM1. The study demonstrates that the biparatopic ADC exhibits superior in vitro and in vivo anti-tumor activity, even against low HER2-expressing and T-DM1-resistant tumors, offering potential for treating a broader range of HER2-related cancers.
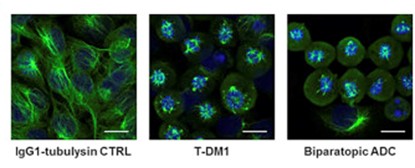 Fig.1 The biparatopic ADC disrupted the intracellular microtubule network in HER2-overexpressing cells.3
Fig.1 The biparatopic ADC disrupted the intracellular microtubule network in HER2-overexpressing cells.3
For more detailed information about construction of biparatopic ADC, please feel free to contact us or directly sent us an inquiry.
Featured Products
Anti-ERBB2/HER2 ADC
| Catalog | Product Name | Antibody |
| ADC-W-116 | Anti-ERBB2 (Trastuzumab)-Gly5-modified DM1 ADC | Humanized Anti-ERBB2 lgG1 antibody, Trastuzumab |
| ADC-W-514 | Anti-ERBB2 (Trastuzumab)-VC-DUBA ADC | Anti-ERBB2 Antibody, Trastuzumab |
| ADC-W-572 | Anti-ERBB2 (Trastuzumab-Fab)-SPP-DM1 ADC | Humanized Anti-ERBB2 lgG1 Antibody, Trastuzumab |
| ADC-W-582 | Anti-ERBB2-SMCC-DM1 ADC | Humanized Anti-ERBB2 lgG1 Antibody, Trastuzumab |
| ADC-W-101 | Anti-ERBB2-Phor18 molecules ADC | lgG1 Anti-ERBB2 lgG1 antibody |
References
- Comer, F.; et al. Bispecific and Biparatopic Antibody Drug Conjugates. Innovations for Next-Generation Antibody-Drug Conjugates (pp. 267-280). Humana Press, Cham. 2018.
- Maruani, A. Bispecifics and antibody-drug conjugates: A positive synergy. Drug Discovery Today: Technologies. 2018, 30: 55-61.
- Li, John Y., et al. "A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy." Cancer cell 29.1 (2016): 117-129.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
