Introduction of PD1
PD-1 (programmed cell death protein 1), also known as CD279 or PDCD1, belongs to the immunoglobulin superfamily of receptors. Encoded by the PDCD1 gene on chromosome 2 in humans, PD-1 consists of an extracellular IgV-like domain, a transmembrane region, and an intracellular tail with two tyrosine-based inhibitory motifs (ITIM and ITSM). Primarily expressed on activated T cells, B cells, NK cells, monocytes, and dendritic cells, PD-1 acts as a negative regulator of immune responses by binding to its ligands, PD-L1 (B7-H1 or CD274) and PD-L2 (B7-DC or CD273). These ligands are expressed on various cell types, including tumor cells and antigen-presenting cells (APCs). The interaction between PD-1 and its ligands inhibits T cell receptor (TCR) signaling, resulting in reduced T cell proliferation, cytokine production, cytotoxicity, and survival, as well as increased T cell exhaustion and apoptosis. While this mechanism maintains peripheral tolerance and prevents excessive immune-mediated tissue damage, it also facilitates tumor immune escape and resistance to immunotherapy.
Introduction of CD137
CD137 (4-1BB), also known as TNFRSF9 or CDw137, belongs to the tumor necrosis factor receptor superfamily (TNFRSF) of receptors. Encoded by the TNFRSF9 gene on chromosome 1 in humans, CD137 consists of an extracellular cysteine-rich domain, a transmembrane region, and an intracellular tail with one TNF receptor-associated factor (TRAF) binding motif. Mainly expressed on activated T cells, NK cells, NKT cells, monocytes, macrophages, dendritic cells, and granulocytes, CD137 serves as a positive regulator of immune responses by binding to its ligand, CD137L (4-1BBL or TNFSF9), expressed on various cell types, including APCs and tumor cells. The interaction between CD137 and its ligand enhances TCR signaling, leading to increased T cell proliferation, cytokine production, cytotoxicity, and survival, as well as reduced T cell exhaustion and apoptosis. This mechanism promotes the expansion and activation of effector T cells and memory T cells, as well as the suppression of regulatory T cells (Tregs), thereby enhancing antitumor immunity.
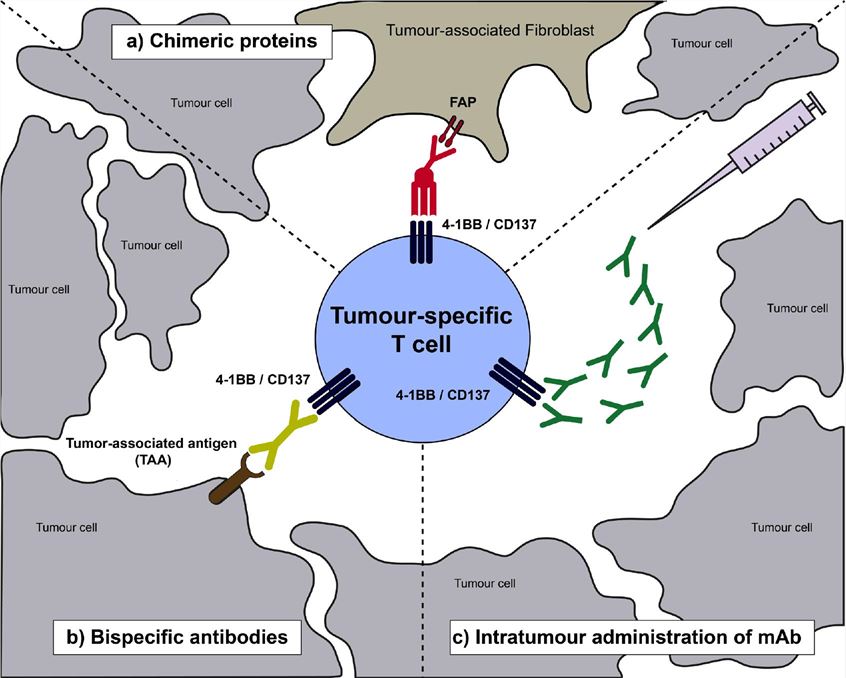
Fig.1 Schematic representation of a tumour-infiltrating T lymphocyte expressing CD137 as a result of priming by tumour-antigen recognition (Etxeberria I, 2020)
Signaling Pathways Involved in Bispecific Antibodies Targeting PD-1 and CD137
The molecular mechanisms of PD-1 and CD137 signaling pathways in T cells are complex and interconnected, involving multiple molecules and cascades that modulate T cell activation and survival. Upon TCR engagement, PD-1 recruits phosphatases such as SHP2, SHP1, SHIP1, and PTEN to its intracellular ITIM and ITSM motifs. These phosphatases dephosphorylate key signaling molecules, including PI3K, Akt, mTOR, Ras, MEK, ERK, NF-kB, JAK, and STAT, inhibiting their downstream effects on T cell function. Conversely, CD137 recruits TRAFs such as TRAF1, TRAF2, and TRAF3 to its intracellular TRAF binding motif. These TRAFs activate kinases such as IKK, NIK, MEKK1, and TAK1, which phosphorylate key signaling molecules, including NF-kB, JNK, p38 MAPK, and ERK. This activation enhances their downstream effects on T cell function. Bispecific antibodies (BsAbs) targeting PD-1 and CD137 simultaneously block the inhibitory PD-1/PD-L1 axis and stimulate the costimulatory CD137/CD137L axis, modulating these signaling pathways synergistically. This dual modulation results in increased T cell proliferation, cytokine production, cytotoxicity, and survival, as well as reduced T cell exhaustion and apoptosis. By overcoming tumor-induced immune suppression, this approach enhances T cell-mediated antitumor activity.
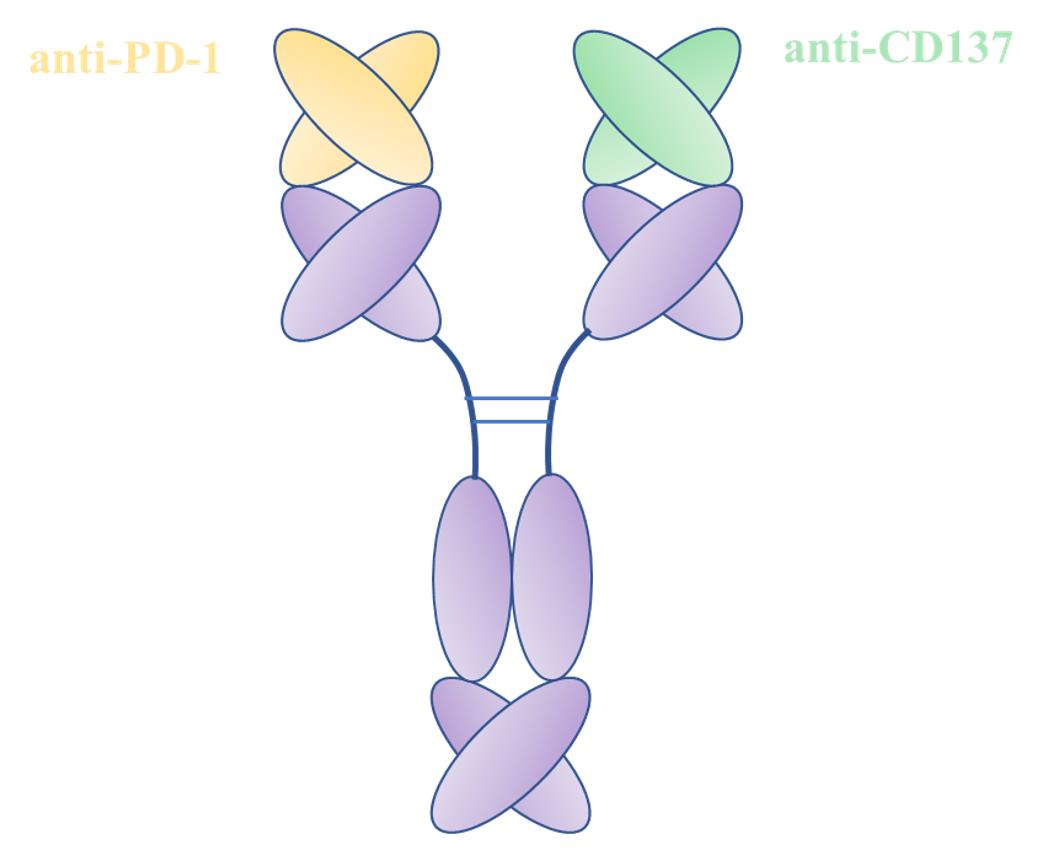
Fig.2 Structure Diagram of BsAb Targeting PD1 and CD137 (Creative Biolabs)
Clinic Status of Bispecific Antibodies Targeting PD-1 and CD137
BsAbs targeting PD1 and CD137 represent a promising new class of immunotherapies with positive preclinical results in various tumor models. However, as of now, no regulatory authority has approved any BsAbs targeting PD1 and CD137 for marketing. Furthermore, their clinical development is in its early stages, with only a few candidates entering clinical trials. Several BsAbs targeting PD1 and CD137 are currently under clinical investigation in different phases and indications. For example, IBI319 is a novel bispecific antibody targeting both PD-1 and CD137, essential molecules in regulating T-cell activation and antitumor immunity.
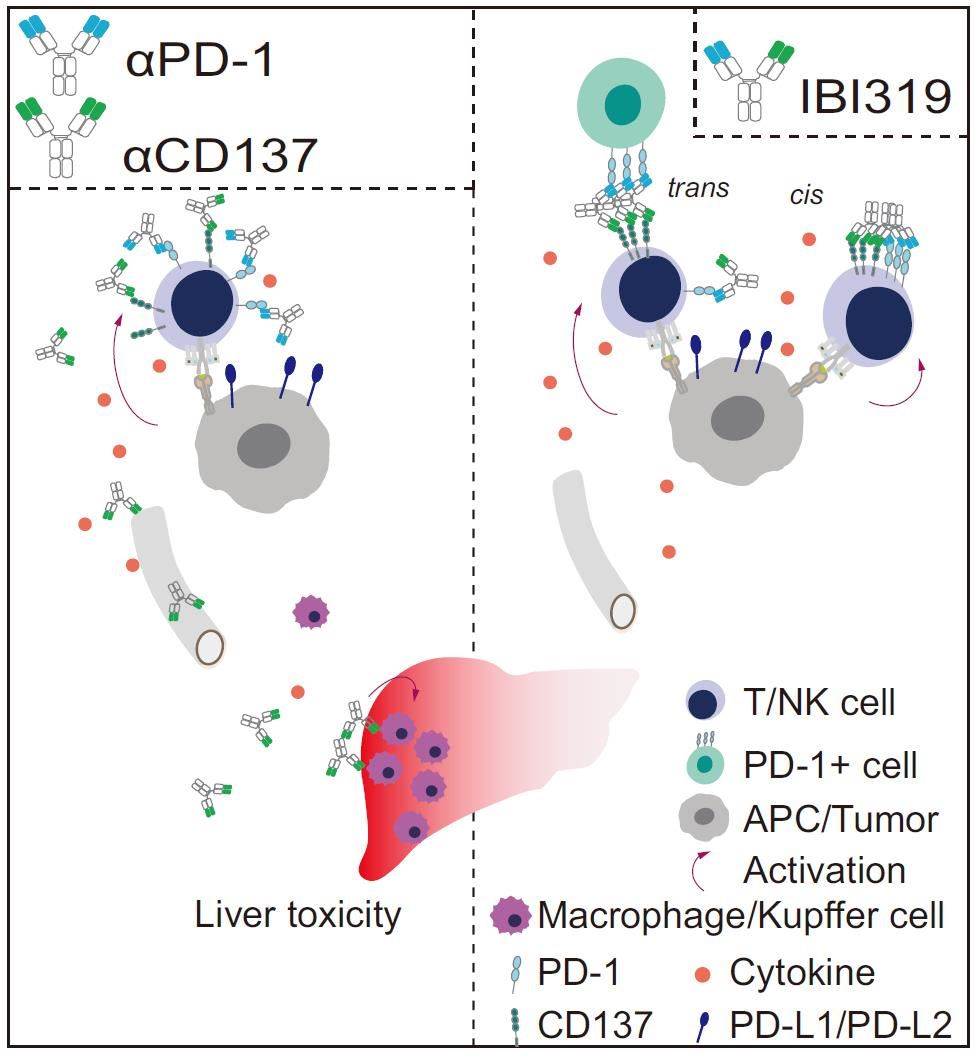
Fig.3 Working Model of IBI319 (Qiao Y, 2021)
By simultaneously blocking PD-1 and activating CD137, IBI319 aims to synergistically stimulate T-cell-mediated antitumor immunity. IBI319 exhibits a higher affinity for PD-1 than for CD137, leading to the preferential accumulation of the antibody on PD-1-high tumor-infiltrating T cells and NK cells, thereby avoiding systemic circulation. Preclinical studies have demonstrated that IBI319 has superior antitumor efficacy and safety compared to the combination of anti-PD-1 and anti-CD137 monoclonal antibodies. Currently under clinical investigation in China for the treatment of advanced solid tumors or hematological malignancies that have failed standard therapy, IBI319 holds promise in advancing the field of immunotherapy.
Table 1. Information of IBI319
|
Trial ID
|
Drug Name
|
Target
|
Sponsor
|
Phase
|
Indication
|
Status
|
|
NCT04140516
|
IBI319
|
PD1 x CD137
|
Innovent Biologics
|
I
|
Solid tumors
|
Recruiting
|
References
1. Etxeberria I, et al. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open. 2020 Jul;4(Suppl 3):e000733.
2. Ugolini A, Nuti M. CD137+ T-Cells: Protagonists of the Immunotherapy Revolution. Cancers (Basel). 2021 Jan 26;13(3):456.
3. Liu G, Luo P. Targeting CD137 (4-1BB) towards improved safety and efficacy for cancer immunotherapy. Front Immunol. 2023 Jun 2;14:1208788.
4. Chu DT, et al. An Update on Anti-CD137 Antibodies in Immunotherapies for Cancer. Int J Mol Sci. 2019 Apr 12;20(8):1822.
5. Puigdelloses M, et al. CD137 and PD-L1 targeting with immunovirotherapy induces a potent and durable antitumor immune response in glioblastoma models. J Immunother Cancer. 2021 Jul;9(7):e002644.
6. Shim H. Bispecific Antibodies and Antibody-Drug Conjugates for Cancer Therapy: Technological Considerations. Biomolecules. 2020 Feb 26;10(3):360.
7. Zhou Y, et al. Immunogenicity assessment of bispecific antibody-based immunotherapy in oncology. J Immunother Cancer. 2022 Apr;10(4):e004225.
8. Qiao Y, et al. Cancer immune therapy with PD-1-dependent CD137 co-stimulation provides localized tumour killing without systemic toxicity. Nat Commun. 2021 Nov 4;12(1):6360.
9. Kim AMJ, et al. 4-1BB: A promising target for cancer immunotherapy. Front Oncol. 2022 Sep 14;12:968360.
10. Zhang T, et al. Bispecific antibodies targeting immunomodulatory checkpoints for cancer therapy. Cancer Biol Med. 2023 Mar 24;20(3):181–95.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY












