Introduction of CD3
CD3 is a protein complex and a co-receptor of the T cell receptor (TCR) that is involved in activating both the cytotoxic T cells (CD8+ naive T cells) and the helper T cells (CD4+ naive T cells). It is composed of four distinct subunits: CD3γ, CD3δ, CD3ε and CD3ζ. CD3 subunits interact with the TCR chains through their transmembrane regions and participate in the surface expression and signal transduction of the TCR after antigen recognition. The cytoplasmic tails of CD3 subunits contain immunoreceptor tyrosine-based activation motifs (ITAMs), which are phosphorylated upon TCR stimulation and recruit ZAP70 kinase, leading to the activation of downstream signaling pathways. CD3 is a key molecule for T cell activation and also a potential target for various immune-related diseases, such as autoimmune diseases, transplant rejection and others.
Introduction of gpA33
gpA33 is a transmembrane glycoprotein belonging to the immunoglobulin superfamily, consisting of a single extracellular domain, a transmembrane region and a cytoplasmic tail. gpA33 is mainly expressed on intestinal epithelial cells, especially in the small intestine and colon. The biological function of gpA33 in normal tissues is not clear, but it may be involved in cell adhesion and signal transduction. gpA33 is overexpressed in more than 95% of colorectal cancers, regardless of tumor differentiation, prognosis and treatment response, making it an ideal colorectal cancer-specific target.
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and gpA33
Bispecific antibodies (BsAbs) targeting CD3 and gpA33 can simultaneously recognize CD3 on T cells and gpA33 on tumor cells, thereby establishing a connection and activation between T cells and tumor cells. BsAbs bridge CD3 and gpA33, triggering the phosphorylation of ITAMs in the CD3 subunits of the TCR complex, recruiting and activating ZAP70 kinase. ZAP70 kinase further activates downstream signaling pathways such as MAPK, NF-κB and PI3K/Akt, leading to T cell proliferation, cytokine secretion (such as IFN-γ, TNF-α, etc.) and cytotoxic release (such as perforin, granzyme, etc.), resulting in tumor cell killing. In addition to the direct cytotoxic effects of BsAbs targeting CD3 and gpA33, some indirect mechanisms may also contribute to the anti-tumor immunity. For example, BsAbs may induce the release of tumor-associated antigens (TAAs) from the killed tumor cells, which can be taken up and presented by antigen-presenting cells (APCs), such as dendritic cells (DCs), to activate more T cells and generate a long-lasting memory response. BsAbs may also modulate the tumor microenvironment (TME) by altering the expression of immune checkpoints, such as PD-1 and PD-L1, on T cells and tumor cells, respectively. Furthermore, BsAbs may recruit other immune cells, such as natural killer (NK) cells and macrophages, to exert additional anti-tumor effects.
Clinic Status of Bispecific Antibodies Targeting CD3 and gpA33
Currently, there are two BsAbs targeting CD3 and gpA33 in clinical trials, namely MGD007 and HuA33-BsAb. MGD007 is a dual-affinity re-targeting protein, composed of two single-chain Fv (scFv) fragments, recognizing CD3ε and gpA33, respectively. MGD007 is developed by MacroGenics and mainly used for the treatment of advanced or metastatic colorectal cancer. MGD007 has completed phase I and Ib/II clinical trials, showing some safety and tolerability, but limited anti-tumor efficacy. HuA33-BsAb is a bispecific antibody (BsAb) targeting CD3 and gpA33 for the treatment of colorectal cancer. It consists of two single-chain Fv (scFv), which recognize CD3ε and gpA33 respectively, can connect T cells and tumor cells, and activate T cells to kill tumor cells. HuA33-BsAb is based on a novel IgG(L)–scFv platform, formed by linking the anti-CD3 huOKT3 scFv to the carboxyl terminus of the light chain.
Table 1. Comparison of HuA33-BsAb and MGD007
|
Property
|
HuA33-BsAb
|
MGD007
|
|
Format
|
IgG (L)-scFv
|
Dual-affinity re-targeting protein
|
|
Target
|
CD3ε and gpA33
|
CD3ε and gpA33
|
|
Developer
|
Memorial Sloan Kettering Cancer Center
|
MacroGenics
|
|
Indication
|
Advanced or metastatic colorectal cancer
|
Advanced or metastatic colorectal cancer
|
|
Clinical trials
|
Phase I ongoing
|
Phase I and Ib/II completed
|
|
Results
|
Not available yet
|
Some safety and tolerability, but limited anti-tumor efficacy
|
|
Mechanism of action
|
Redirects T cells to kill gpA33-positive tumor cells
|
Redirects T cells to kill gpA33-positive tumor cells
|
|
Dose and administration
|
Not available yet
|
Intravenous infusion, once every 2 weeks
|
|
Adverse events
|
Not available yet
|
Cytokine release syndrome, infusion-related reactions, fatigue, nausea, diarrhea, etc.
|
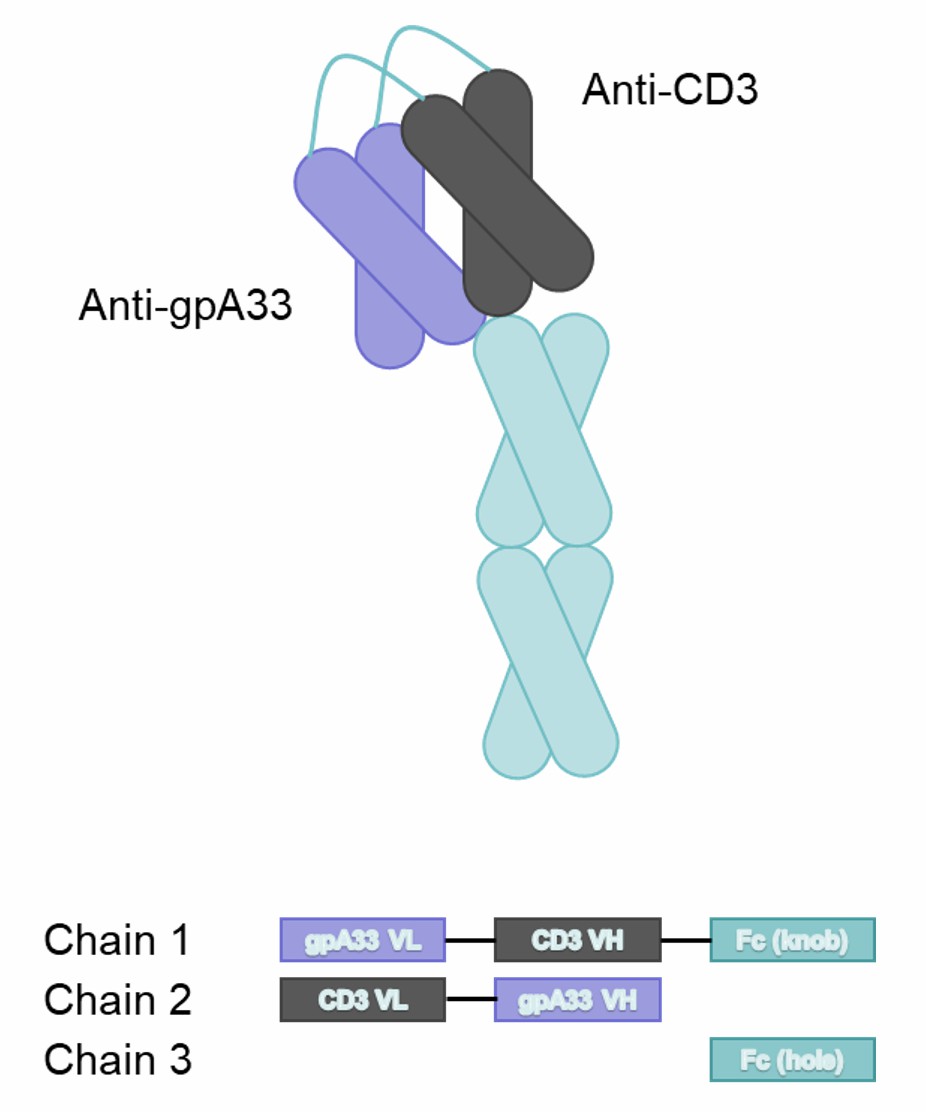
Fig.1 MGD007 Consisting of Three Chains Covalently Linked by Disulfide Bonds (Creative Biolabs)
References
1. Wu Z, et al. Development of a Tetravalent Anti-GPA33/Anti-CD3 Bispecific Antibody for Colorectal Cancers. Mol Cancer Ther. 2018 Oct;17(10):2164-2175.
2. Farhangnia P, et al. Bispecific Antibodies in Targeted Cancer Immunotherapy. In: Handbook of Cancer and Immunology. Springer; 2023.
3. Jin C, et al. Bispecific antibodies as a development platform for new concepts and treatment strategies. Int J Mol Sci. 2017 Jan 6;18(1):48.
4. Ma Y, et al. Development of bispecific antibodies and their applications in tumor immunotherapy. Expert Opin Biol Ther. 2019 Jan;19(1):1-19.
5. Nisonoff A, et al. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Nov;89:230-244.
6. Cheung NV, et al. Targeting CD3 with bispecific antibodies for cancer therapy. Cancer Treat Res Commun. 2020;23:100168.
7. Staerz UD, et al. Hybrid antibodies can target sites for attack by T cells. Nature. 1985 Feb 28-Mar 6;313(6005):786-788.
8. Wu Z, Cheung NV. T cell engaging bispecific antibody (T-BsAb): From technology to therapeutics. Pharmacol Ther. 2019 Mar;195:1-25.
9. Moore PA, et al. Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood. 2011 Jun 2;117(22):6043-6054.
10. Cunningham D, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004 Jul 22;351(4):337-345.
11. Amado RG, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008 Apr 1;26(10):1626-1634.
12. Karapetis CS, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008 Oct 23;359(17):1757–1765.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










