Introduction to CD3
CD3 is a complex of four distinct subunits (γ, δ, ε, and ζ) that associate with the T cell receptor (TCR) and are essential for TCR expression and signal transduction. CD3 is encoded by five genes (CD3G, CD3D, CD3E, CD247, and CD3Z) located on chromosome 11 in humans. The CD3 subunits have a single extracellular immunoglobulin-like domain, a transmembrane region with charged amino acids that interact with the TCR chains, and an intracellular tail with one or more immunoreceptor tyrosine-based activation motifs (ITAMs) that are phosphorylated upon TCR engagement by antigen-presenting cells. CD3 is expressed on all mature T cells and plays a crucial role in T cell development, activation, differentiation, and function. CD3 is also a potential target for immunotherapy of various diseases, such as autoimmune disorders, transplant rejection, viral infections, and cancer.
Introduction to PSMA
PSMA is a type II transmembrane glycoprotein that belongs to the glutamate carboxypeptidase family. PSMA is encoded by the FOLH1 gene located on chromosome 11 in humans. PSMA has a large extracellular domain with metallopeptidase activity that cleaves various substrates involved in folate metabolism, glutamate signaling, angiogenesis, and cell invasion. PSMA also has a short intracellular domain with two tyrosine residues that can be phosphorylated and interact with signaling molecules. PSMA is normally expressed in low levels on the epithelial cells of the prostate gland and other tissues, such as the small intestine, kidney, brain, and salivary glands. However, PSMA expression is significantly upregulated in prostate cancer cells and correlates with tumor grade, stage, metastasis, recurrence, and resistance to hormone therapy. PSMA is also expressed on tumor-associated neovasculature of various solid tumors. Therefore, PSMA is an attractive target for the diagnosis and treatment of prostate cancer and other malignancies.
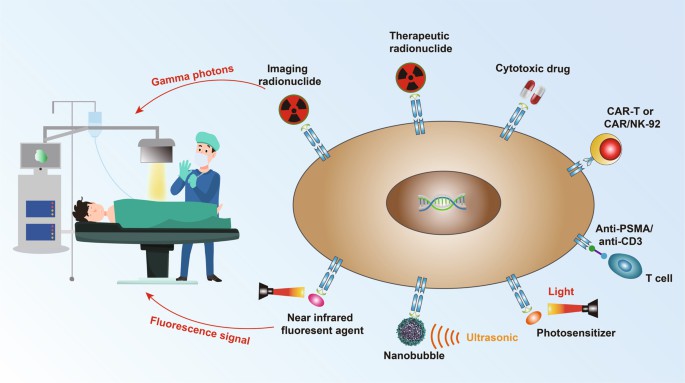
Fig.1 Tumor therapies targeting PSMA (Wang F, 2022)
Signaling Pathways Involved in Bispecific Antibodies Targeting CD3 and PSMA
The mechanism of action of CD3/PSMA bsAbs involves the cross-linking of CD3 on T cells and PSMA on tumor cells, resulting in the formation of an immunological synapse and the activation of multiple signaling pathways that mediate T cell proliferation, cytokine production, cytotoxicity, and memory formation. CD3/PSMA bsAbs cross-link CD3 on T cells and PSMA on tumor cells, resulting in the phosphorylation of ITAMs on CD3 subunits by SFKs. This leads to the recruitment of ZAP70 and the activation of downstream signaling cascades, such as MAPKs, NF-κB, and Ca2+/NFAT pathways. These pathways regulate the expression of genes involved in T cell activation, differentiation, and function. Costimulatory receptors on T cells interact with their ligands on antigen-presenting cells or tumor cells and enhance TCR/CD3 signaling by activating various signaling molecules, such as PI3K/AKT/mTOR, TRAFs/NF-κB/MAPKs/JNKs. These molecules regulate the expression of genes involved in T cell proliferation, cytokine production, cytotoxicity, and memory formation. Inhibitory receptors on T cells interact with their ligands on antigen-presenting cells or tumor cells and inhibit TCR/CD3 and costimulatory signaling by recruiting phosphatases that dephosphorylate key signaling molecules. This results in the inhibition of T cell activation, proliferation, cytokine production, cytotoxicity, and survival.
Clinical Status of Bispecific Antibodies Targeting CD3 and PSMA
Several CD3/PSMA bsAbs are being developed for the treatment of prostate cancer by different companies or research institutions. Some of them are currently undergoing clinical trials in patients with mCRPC who have failed or are resistant to standard therapies.
Table 1. Clinical status of CD3/PSMA bsAb in prostate cancer.
|
Name
|
Format
|
Developer
|
Clinical Trial
|
Phase
|
Status
|
|
AMG 160
|
BiTE
|
Amgen
|
NCT03792841
|
I
|
Recruiting
|
AMG 160 is one of the most advanced CD3/PSMA bsAbs, a half-life extended BiTE (bispecific T cell engager) molecule that consists of two single-chain variable fragments (scFvs) linked by a flexible peptide linker. AMG 160 is designed to bind to CD3ε on T cells and PSMA on tumor cells with high affinity and specificity, and to induce potent T cell-mediated cytotoxicity against PSMA-positive prostate cancer cells. REGN5678 is a full-length IgG1 antibody that has two anti-CD3 scFvs fused to its heavy chains and two anti-PSMA scFvs fused to its light chains. REGN5678 is designed to have a similar structure and function as natural IgG antibodies, and to induce T cell activation and tumor killing by cross-linking CD3 on T cells and PSMA on tumor cells.
There are other CD3/PSMA bsAbs that are in preclinical or early clinical development. These CD3/PSMA bsAbs have different formats, such as BiTEs, DARTs (dual-affinity re-targeting), TriTACs (trispecific T cell-activating constructs), IgGs, and ADCs (antibody-drug conjugates), and may have different pharmacological properties, such as half-life, stability, affinity, specificity, potency, and toxicity. The clinical development of these CD3/PSMA bsAbs will provide more insights into the optimal design and application of this novel class of immunotherapeutic agents for prostate cancer.
References
1. Bacac M, et al. CEA TCB: A novel head-to-tail 2:1 T cell bispecific antibody for treatment of CEA-positive solid tumors. Oncoimmunology. 2016 Sep 2;5(10):e1203498.
2. Wang F, et al. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2022 Mar;25(1):11-26.
3. Staerz UD, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature. 1986 Feb 13-19;319(6055):628-31.
4. Bargou R, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008 Aug 15;321(5891):974-7.
5. Seimetz D, et al. Development and approval of the trifunctional antibody catumaxomab (anti-EpCAM x anti-CD3) as a targeted cancer immunotherapy. Cancer Treat Rev. 2010 Oct;36(6):458-67.
6. Baeuerle PA, Reinhardt C. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009 Jun 15;69(12):4941-4.
7. Dreier T, et al. Extremely potent, rapid and costimulation-independent cytotoxic T-cell response against lymphoma cells catalyzed by a single-chain bispecific antibody. Int J Cancer. 2002 Jul 10;100(2):690-7.
8. Loffler A, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood. 2000 Mar 15;95(6):2098-103.
9. Offner S, et al. Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol. 2006 Jul;43(10):763-71.
10. Wu J, et al. Blinatumomab: a bispecific T cell engager (BiTE) antibody against CD19/CD3 for refractory acute lymphoid leukemia. J Hematol Oncol. 2015 Nov 25;8:104.
11. Viardot A, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016 Mar 24;127(12):1410-6.
12. Topp MS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015 Jan;16(1):57-66.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










