What is Fab-scFv
Fab-scFv is a bispecific antibody composed of a Fab fragment and an scFv fragment connected by a heterologous Fc region. The Fab fragment retains the variable and constant domains of the first structural domain of a complete antibody, with high affinity and specificity. The scFv fragment consists of a single-chain variable region, which connects the variable regions of the heavy and light chains with a linker peptide. Compared with full-length antibodies or other bispecific antibody formats, Fab-scFv has advantages such as targeting two different antigens or epitopes simultaneously, avoiding light chain mispairing, extending half-life, activating or inhibiting immune effector functions, and better penetrating into tumor tissues. However, it also has limitations, such as scFv fragments may cause antibody aggregation or denaturation, recombinant DNA technology or phage display technology may involve technical difficulties, high costs, low efficiency and other problems, and may cause immunogenicity or toxicity reactions.
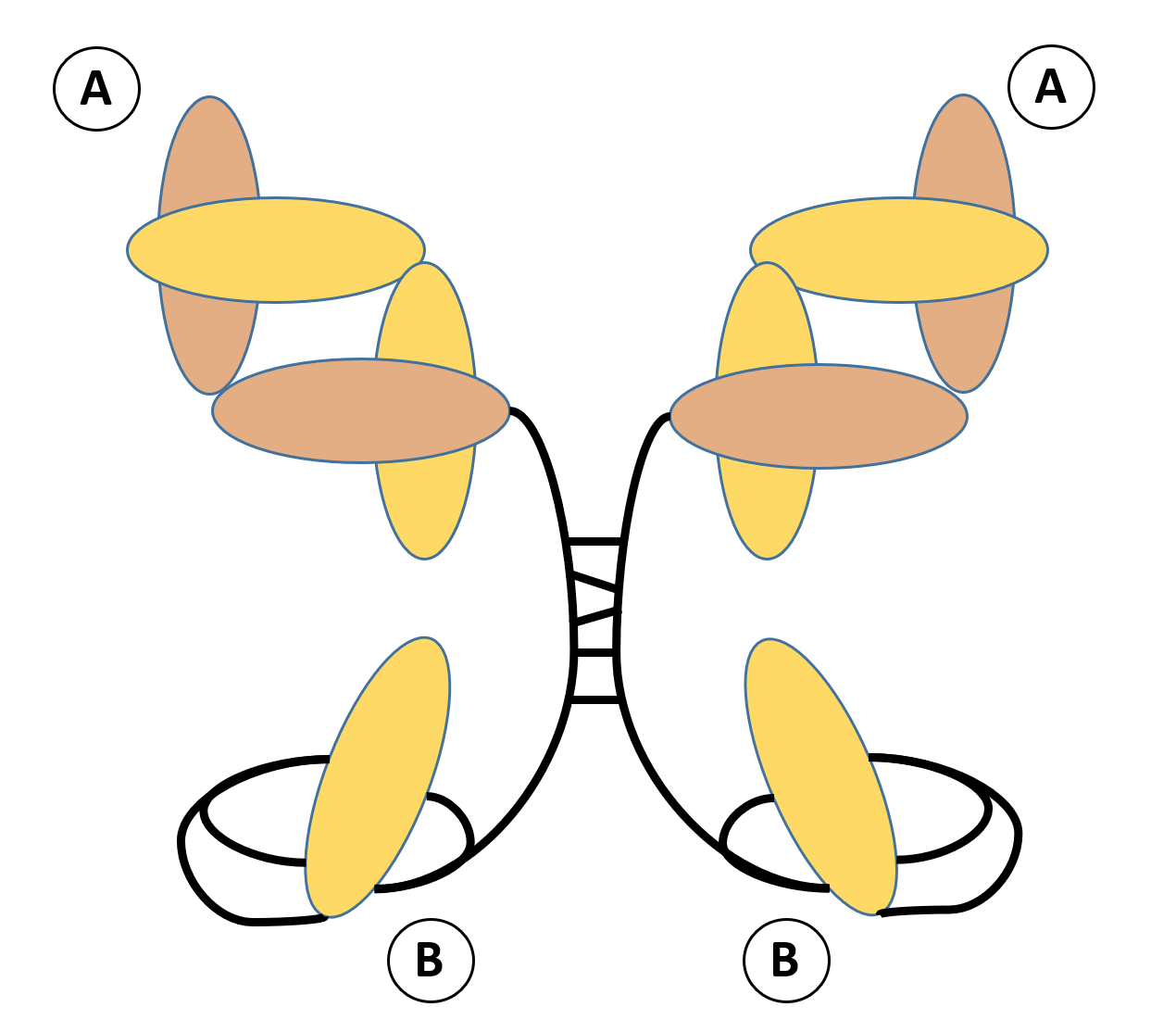
Fig.1 Schematic representation of Fab-scFv homodimer.
Fab-scFv has a broad application prospect in cancer treatment, and can achieve various functions such as immune cell redirection, multi-target or multi-epitope recognition, immune checkpoint modulation and so on. At present, several Fab-scFv have entered clinical trials or market applications, showing good efficacy and feasibility. This article will introduce and analyze Fab-scFv's structural characteristics, generation methods, clinical data and other aspects in detail.
Fab-scFv Generation Methods
Fab-scFv is produced by recombinant DNA technology or phage display technology. Recombinant DNA technology is to use DNA recombinase to link the genes of Fab fragment and scFv fragment together, and then transfect them into host cells, such as Escherichia coli, yeast or mammalian cells, to express Fab-scFv protein. Phage display technology is to use phage as a vector, and insert the genes of Fab fragment and scFv fragment into the phage coat protein genes, and then infect them into Escherichia coli, to express Fab-scFv fusion protein, and purify it by affinity chromatography and other methods.
Recombinant DNA technology and phage display technology have their own advantages and disadvantages. The advantage of recombinant DNA technology is that it can produce high levels of Fab-scFv protein, and its stability and activity can be improved by optimizing the host cells and culture conditions. The disadvantage of recombinant DNA technology is that it requires the construction of transfection vectors, which may involve cloning, screening, verification and other steps, which are time-consuming and labor-intensive. In addition, recombinant DNA technology may also have problems such as low expression efficiency, difficult purification, inconsistent glycosylation and so on. The advantage of phage display technology is that it can quickly construct large-scale Fab-scFv libraries, and find high-affinity Fab-scFv candidates by affinity screening. The disadvantage of phage display technology is that the Fab-scFv fusion protein produced may have the risk of aggregation or denaturation, and it needs an additional expression system to produce soluble Fab-scFv protein.
Clinical Data of Fab-scFv
Fab-scFv is an innovative bispecific antibody format that has advantages such as smaller molecular size, higher tissue penetration, lower immunogenicity and easier engineering, which make it have great potential and competitiveness in the market. So far, two Fab-scFv have been approved by the US Food and Drug Administration (FDA), namely ReoPro® and Lumoxiti®.
Table.1 Fab-scFv in market applications
|
Product name
|
Fab-scFv name
|
Target
|
Indication
|
Developer
|
Marketer
|
|
ReoPro®
|
Abciximab
|
GPIIb/IIIa
|
Acute coronary syndrome
|
Centocor Inc.
|
Eli Lilly and Company
|
|
Lumoxiti®
|
Moxetumomab pasudotox-tdfk
|
CD22
|
Hairy cell leukemia
|
MedImmune Inc.
|
AstraZeneca plc
|
Currently, several Fab-scFv have entered clinical trial stages, mainly for different types of cancers, such as breast cancer, ovarian cancer, lung cancer, gastric cancer and so on. Table 2 lists some information of ongoing or completed Fab-scFv-related clinical trials, including their targets, diseases, and sponsors.
Table.2 Fab-scFv in clinical trials
|
Clinical trial number
|
Fab-scFv name
|
Target
|
Disease
|
Sponsor
|
|
NCT04187922
|
MGD019
|
PD-1×CTLA-4
|
Solid tumors
|
MacroGenics
|
|
NCT04322118
|
MGD019
|
PD-1×CTLA-4
|
Non-small cell lung cancer
|
MacroGenics
|
|
NCT04661374
|
MGD019
|
PD-1×CTLA-4
|
Gastric/esophageal cancer
|
MacroGenics
|
|
NCT03761056
|
MGD007
|
GP-A33×CD3ε
|
Gastrointestinal cancer
|
MacroGenics
|
|
NCT03698994
|
MGD007
|
GP-A33×CD3ε
|
Colorectal cancer
|
MacroGenics
|
|
NCT03219268
|
MGC018
|
B7-H3×Duocarmycin SA
|
Solid tumors
|
MacroGenics
|
|
NCT04661361
|
MGC018
|
B7-H3×Duocarmycin SA
|
Prostate cancer
|
MacroGenics
|
|
NCT04661348
|
MGC018
|
B7-H3×Duocarmycin SA
|
Breast/ovarian/endometrial, etc.
|
MacroGenics
|
|
NCT04187935
|
MGD013
|
PD-1×LAG-3
|
Solid tumors
|
MacroGenics
|
|
NCT04201849
|
MGD013
|
PD-1×LAG-3
|
Non-small cell lung cancer
|
MacroGenics
|
|
NCT04661335
|
MGD013
|
PD-1×LAG-3
|
Breast/ovarian/endometrial, etc.
|
MacroGenics
|
|
NCT03761069
|
MGD009
|
B7-H3×CD3ε
|
Solid tumors
|
MacroGenics
|
|
NCT04322105
|
MGD009
|
B7-H3×CD3ε
|
Non-small cell lung cancer
|
MacroGenics
|
|
NCT04322092
|
MGD009
|
B7-H3×CD3ε
|
Gastric/esophageal cancer
|
MacroGenics
|
|
NCT03761043
|
MGD006
|
CD123×CD3ε
|
Acute myeloid leukemia
|
MacroGenics
|
|
NCT03761030
|
MGD006
|
CD123×CD3ε
|
Myelodysplastic syndrome
|
MacroGenics
|
|
NCT03761140
|
MGD006
|
CD123×CD3ε
|
Long-term survivors of acute myeloid leukemia (≥6 months)
|
MacroGenics
|
|
NCT03761127
|
MGD006
|
CD123×CD3ε
|
Long-term survivors of myelodysplastic syndrome (≥6 months)
|
MacroGenics
|
|
NCT03761114
|
MGD006
|
CD123×CD3ε
|
Acute lymphoblastic leukemia or lymphoma patients (≥6 months)
|
MacroGenics
|
References
1. Nesspor TC, et al. High-Throughput Generation of Bipod (Fab × scFv) Bispecific Antibodies Exploits Differential Chain Expression and Affinity Capture. Sci Rep. 2020 May 5;10(1):7557.
2. Kipriyanov SM, Le Gall F. Generation and production of engineered antibodies. Mol Biotechnol. 2004 Jan;26(1):39-60.
3. Crivianu-Gaita V, et al. Aptamers, antibody scFv, and antibody Fab' fragments: An overview and comparison of three of the most versatile biosensor biorecognition elements. Biosens Bioelectron. 2016 Nov 15;85:32-45.
4. Holliger P, et al. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005 Sep;23(9):1126-36.
5. Knight DM, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993 Dec;30(16):1443-53.
6. Valgimigli M, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention with stent implantation: an analysis from the EPISTENT trial. Eur Heart J. 2008 Jan;29(2):218-25.
7. Eli Lilly and Company. ReoPro® (abciximab) Injection for Intravenous Use [package insert]. Indianapolis, IN: Eli Lilly and Company; 2018.
8. MedImmune Inc. Lumoxiti® (moxetumomab pasudotox-tdfk) Injection for Intravenous Use [package insert]. Gaithersburg, MD: MedImmune Inc.; 2018.
9. AstraZeneca plc. Lumoxiti® (moxetumomab pasudotox-tdfk) Injection for Intravenous Use [product information]. Cambridge, UK: AstraZeneca plc; 2021.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY





