Antibodies are a class of biomolecules that have high specificity and affinity for recognizing and binding to specific antigens, thereby exerting immunomodulatory and therapeutic effects. With the development of biotechnology, people have continuously modified and optimized antibodies to improve their potency and safety, and expand their application scope. Bispecific antibodies (BsAbs) are a class of antibodies that can simultaneously recognize two different antigens or epitopes, and can achieve various novel mechanisms of action, such as dual blockade, dual activation, and cell redirection. BsAbs have broad application prospects in the fields of tumor, infection, and autoimmunity.
scDiabody-Fc is a novel BsAb format, consisting of two single-chain bispecific antibody fragments (scDiabodies) and an Fc region. scDiabody is a bispecific antibody fragment composed of two single-chain variable fragments (scFvs) connected by a linker peptide, each scFv recognizing a different antigen. The Fc region is a structural domain composed of two heavy chain constant regions, which can enhance the stability, half-life and effector functions of scDiabody. scDiabody-Fc has 2+2 antigen-binding valency, and can simultaneously recognize and regulate two different targets, enhancing the affinity and specificity of the antibody.
Structural Features of scDiabody-Fc
scDiabody-Fc is a fusion protein composed of two scDiabodies and an Fc region, with 2+2 antigen-binding valency. scDiabody is a bispecific antibody fragment composed of two single-chain variable fragments (scFvs) connected by a linker peptide, each scFv recognizing a different antigen. scFv is a single-chain antibody fragment composed of a variable heavy chain (VH) and a variable light chain (VL) connected by a short peptide, with the same antigen-binding site as natural antibodies. scDiabody has the advantages of smaller molecular weight (about 50 kDa), higher stability and easier expression compared to scFv. The three-dimensional structure of scDiabody usually presents as a double-loop shape, each loop consisting of a VH and a VL, and the two loops connected by a linker peptide. The length and sequence of the linker peptide have a great influence on the stability and activity of scDiabody, and need to be optimized. Generally speaking, the shorter the linker peptide, the better, to avoid excessive flexibility and entropy loss. Common linker peptide sequences are (Gly4Ser)n, (Gly3Ser)n, (Gly2Ser)n, etc., where n is the number of repetitions.
The Fc region is a structural domain composed of two heavy chain constant regions, which can enhance the stability, half-life and effector functions of scDiabody. The Fc region can bind to serum albumin (HSA) or neobiotin (NIB), prolonging the circulation time of antibodies in vivo. The Fc region can also bind to Fc receptors (FcRs) or complement, activating antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC), thereby enhancing the killing effect of antibodies on target cells. An scDiabody-Fc with an Fc region can recognize two different antigens, A and B, and bind to FcR or complement. The three-dimensional structure of the Fc region usually presents as a Y-shape, with each arm consisting of a CH2 and a CH3, and the two arms connected by a disulfide bond. Figure 2 shows a schematic diagram of an scDiabody-Fc with an Fc region that can recognize two different antigens, A and B, and bind to FcR or complement.
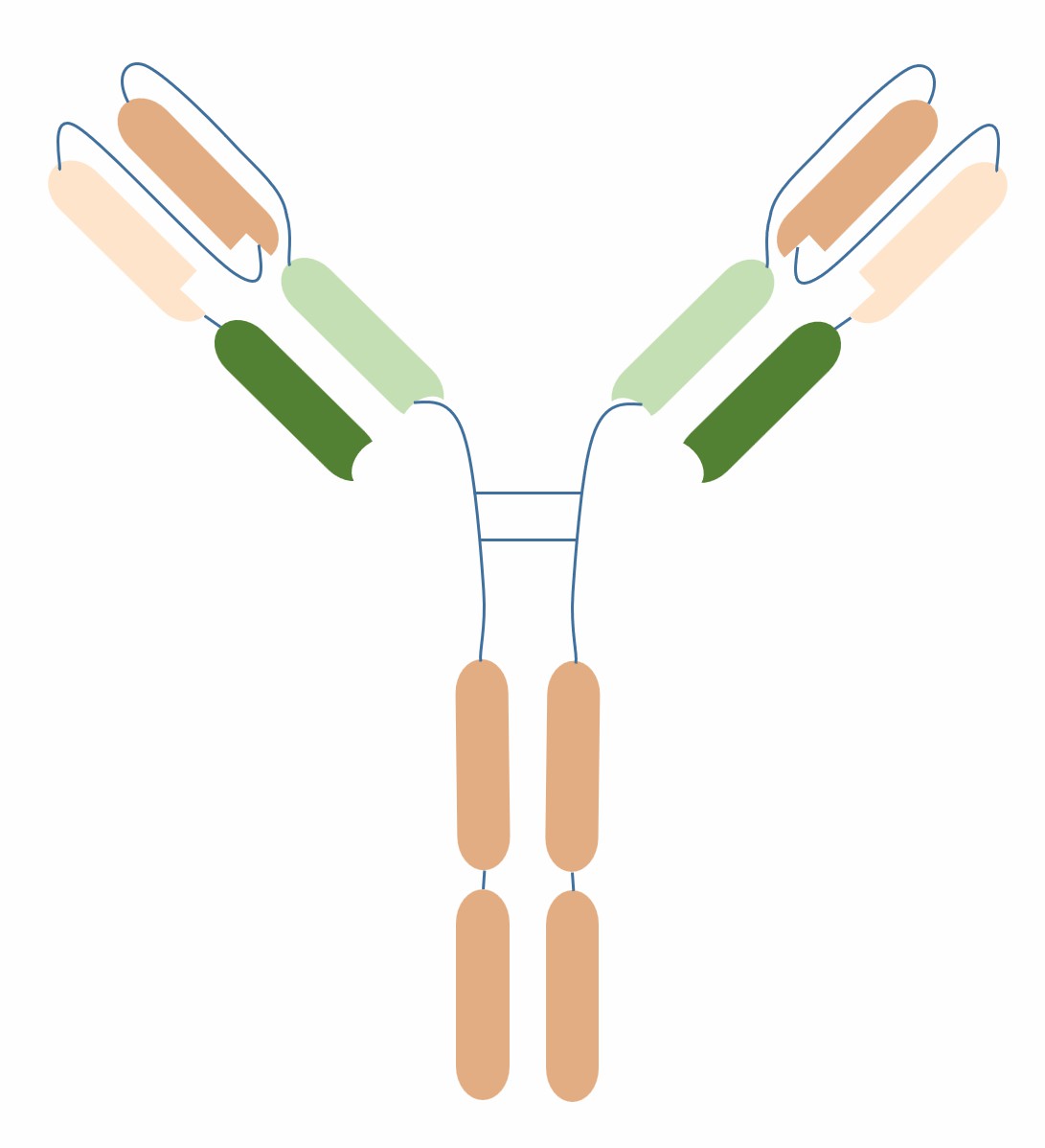
Fig.1 Schematic diagram of the structure of scDiabody-Fc (Creative Biolabs)
Clinical Data of scDiabody-Fc
scDiabody-Fc is a novel bispecific antibody fusion protein, with the advantages of simultaneously recognizing and regulating two different targets, enhancing the affinity and specificity of the antibody, and utilizing Fc-mediated effector functions. scDiabody-Fc has potential therapeutic value in various disease areas, such as hematological malignancies, solid tumors, infections, autoimmunity, etc. Some scDiabody-Fc have entered clinical trials.
Table 1. Information of scDiabody-Fc in clinical trials
|
Targets
|
Company/Institution
|
Clinical Trial Phase
|
Indication
|
Registration Number
|
|
FIXa & FX
|
Creative Biolabs
|
Preclinical
|
Hemophilia A
|
-
|
|
CD16 & HER2
|
Creative Biolabs
|
Preclinical
|
Breast cancer, gastric cancer, prostate cancer, ovarian cancer, solid tumors
|
-
|
|
Ang2 & VEGFA
|
Creative Biolabs
|
Preclinical
|
Solid tumors
|
-
|
|
CD3 & BCMA
|
Molecular Templates, Inc.
|
Phase 1/2
|
Relapsed or refractory multiple myeloma (MM)
|
NCT04585750
|
|
CD3 & CD20
|
Molecular Templates, Inc.
|
Phase 1/2
|
Relapsed or refractory B-cell malignancies (B-NHL) or chronic lymphocytic leukemia (CLL)
|
NCT04590430
|
So far, no scDiabody-Fc has been approved for marketing, but some similar bispecific antibody fusion proteins have been approved, such as Blincyto (CD3 & CD19) and Empliciti (SLAMF7 & FcγRIIIa).
Table 2. Information of bispecific antibody fusion proteins that have been marketed
|
Targets
|
Indication
|
Population
|
Country/Region
|
|
CD3 & CD19
|
Relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL)
|
Patients aged ≥3 years old
|
USA, EU, Japan, etc.
|
|
SLAMF7 & FcγRIIIa
|
Relapsed or refractory multiple myeloma (MM)
|
Patients treated with Revlimid and dexamethasone
|
USA, EU, Japan, etc.
|
References
1. Wang Q, et al. Design and Production of Bispecific Antibodies. Antibodies (Basel). 2019 Aug 2;8(3):43.
2. Labrijn AF, et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019 Aug;18(8):585-608.
3. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
4. Kontermann RE, et al. Bispecific antibodies. Drug Discov Today. 2010 Jun;15(11-12): 505-19.
5. Brinkmann U, et al. The making of bispecific antibodies. MAbs. 2017 Jan;9(1):182-212.
6. Lewis SM, et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol. 2014 Feb;32(2):191-8.
7. Wu C, et al. Engineering monovalent bispecific and trispecific antibodies with novel mechanisms of action using CrossMab technology. MAbs. 2017 Jul;9(5):741-53.
8. Schaefer W, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific IgG antibodies. Proc Natl Acad Sci U S A. 2011 Jul 19;108(29):11187-92.
9. Spiess C, et al. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat Biotechnol. 2013 Jul;31(7):647-9.
10. Klein C, et al. Progress in overcoming the chain association issue in bispecific heterodimeric IgG antibodies. MAbs. 2009 Nov-Dec;1(6):532-40.
11. Wu X, et al. Fc engineering for developing therapeutic bispecific antibodies and novel scaffolds. Front Immunol . 2017 Sep 27;8:38.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










