Background of Triple Body
Antibody drugs represent a class of biologics that employ antibodies or their derivatives as active ingredients to prevent or treat human or animal diseases. In fields like cancer, autoimmune diseases, and infectious diseases, antibody drugs have emerged as top-selling pharmaceuticals, demonstrating promising clinical applications. However, they still face certain challenges and obstacles. As a result, continuous exploration of novel technologies and strategies is essential to enhance the quality and efficiency of antibody drugs. Triple body, a cutting-edge multifunctional antibody platform, is composed of three single-chain variable fragments (scFvs), each with distinct targeting specificity, enabling simultaneous recognition of three different antigens or receptors. Its structural design and expression mode differ from traditional bispecific antibodies (BsAbs) and trispecific antibodies (TsAbs), providing enhanced flexibility and stability. Various methods, such as gene recombination, chemical coupling, and enzyme catalysis, can be employed to generate triple body. While triple body offers several advantages in clinical applications, such as the activation of multiple immune cells, improved tumor-killing effects, and expanded therapeutic potential, it also present some limitations, including potential increases in toxicity, immunogenicity, and production difficulties.
Structural Features and Generation Methods of Triple Body
Triple body is a trivalent antibody composed of three single-chain variable fragments (scFvs), each with a unique targeting specificity, enabling simultaneous recognition of three different antigens or receptors. scFv, the smallest antibody unit composed of heavy and light chain variable regions connected by a peptide linker, ensures high stability and expression efficiency. The three scFvs in triple body can be combined in different arrangements and connection modes, leading to various types of triple bodies, such as tandem, parallel, Y-shaped, T-shaped, among others. The structural design and expression mode of triple body differ significantly from traditional bispecific antibodies (BsAbs) and trispecific antibodies (TsAbs), providing heightened flexibility and stability.
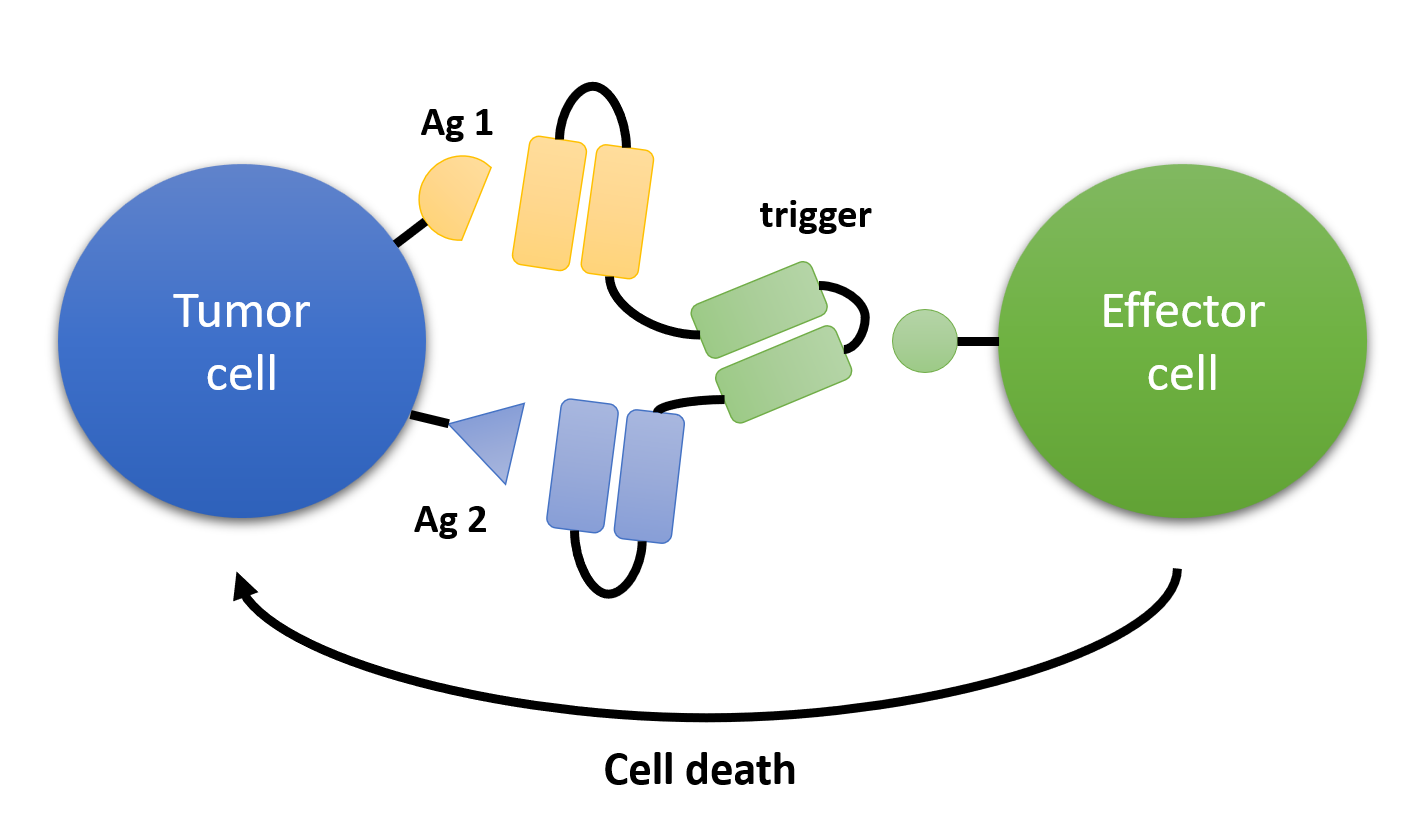
Fig.1 Triple Bodies for the Treatment of Cancer
Triple body can be generated through diverse methods, including gene recombination, chemical coupling, and enzyme catalysis. The gene recombination method involves using molecular biology technology to connect the three scFv genes into a single expression vector, which is then expressed and purified in the desired triple body by transfecting host cells. The chemical coupling method employs specific chemical reactions to introduce functional groups on the three scFv molecules, leading them to undergo cross-linking reactions under appropriate conditions, forming stable covalent bonds, and producing the desired triple body. Lastly, the enzyme catalysis method utilizes enzyme-catalyzed reactions to introduce specific substrates or coenzymes on the three scFv molecules, causing them to undergo transfer or condensation reactions under the action of enzymes, thus forming stable covalent bonds and resulting in the desired triple body.
Clinical applications of Triple Body
Triple body, as a trivalent antibody with multiple targeting specificities, holds substantial potential in cancer immunotherapy by simultaneously recognizing and eliminating three different cell types. Currently, three triple body drugs have been approved worldwide, namely Emabody and Trixobody, primarily targeting hematological malignancies and achieving potent tumor-killing effects by simultaneously activating T cells, NK cells and recognizing tumor-associated antigens. These drugs are indicated for B-cell acute lymphoblastic leukemia, Hodgkin lymphoma and multiple myeloma, primarily for relapsed or refractory patients. The introduction of these drugs has introduced new treatment options in the clinic and provided crucial data and experience for the development of triple bodies.
Table 1. Approved Triple Body Drugs
|
Drug name
|
Targets
|
Approval date
|
Indications
|
Population
|
Country/Region
|
|
Emabody
|
CD3/CD19/CD22
|
January 2022
|
B-cell acute lymphoblastic leukemia
|
Children and adolescents
|
USA
|
|
Trixobody
|
CD3/CD20/CD38
|
December 2020
|
Multiple myeloma
|
Relapsed or refractory patients
|
China
|
At present, numerous triple body drugs are undergoing clinical trials at different stages, covering various diseases such as acute myeloid leukemia, solid tumors, HIV infection, non-Hodgkin lymphoma and chronic lymphocytic leukemia. The target selection of these drugs is also diverse and innovative, including HER2, EGFR, CCR5, CD37 and CD47. The clinical trial data of these drugs will provide additional evidence and support for the safety, efficacy and mechanisms of triple body.
Table 2. Triple Body Drugs in Clinical Trials
|
Drug name
|
Targets
|
Clinical trial phase
|
Indications
|
Trial number
|
|
Triplify
|
CD3/CD33/CD123
|
Phase I
|
Acute myeloid leukemia
|
NCT04678925
|
|
Triplizumab
|
CD3/HER2/EGFR
|
Phase I/II
|
Solid tumors
|
NCT04123470
|
|
Tripligan
|
CD3/CD4/CCR5
|
Phase II
|
HIV infection
|
NCT04270251
|
|
Triplituximab
|
CD3/CD20/CD37
|
Phase I
|
Non-Hodgkin lymphoma
|
NCT04330542
|
|
Tripliruximab
|
CD3/CD20/CD47
|
Phase I
|
Non-Hodgkin lymphoma and chronic lymphocytic leukemia
|
NCT04474470
|
References
1. Hoffmann C, et al. Triplebodies: a new class of antibody-derived protein therapeutics. Methods Mol Biol. 2014;1060:151-66.
2. Braciak TA, et al. NK cells from an AML patient have recovered in remission and reached comparable cytolytic activity to that of a healthy monozygotic twin mediated by the single-chain triplebody SPM-2. J Transl Med. 2013 Nov 16;11:289.
3. Liu Y, et al. Triple body: a novel bispecific antibody format for cancer immunotherapy. BioDrugs. 2020 Aug;34(4):413-423.
4. Kellner C, et al. Triplebody Mediates Increased Anti-Leukemic Reactivity of Natural Killer Cells. Transfus Med Hemother. 2012 Oct;39(5):353-60.
5. Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017 Mar 2;376(9):836-847.
6. Kellner C, et al. A novel CD16/CD30 bispecific antibody redirects NK-cell cytotoxicity towards Hodgkin's lymphoma cells and improves ADCC by preloading FcγRIIIa with CD30L. Oncoimmunology. 2016 Aug 25;5(10):e1226720.
7. Herrmann M, et al. First-in-human phase I study of the B-cell maturation antigen-directed triple body AMG 701 in relapsed/refractory multiple myeloma. Blood Cancer J. 2021 Jan 8;11(1):7.
8. Klinger M, et al. A novel CD19-directed triple body with enhanced tumor killing capacity and improved pharmacokinetics for treatment of B-lineage malignancies. MAbs. 2019 Jan;11(1):69-81.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










