Bispecific antibodies (BsAbs) are antibody molecules that can simultaneously recognize and bind two different antigens or epitopes, with a wide range of biological and clinical applications. BsAbs can be classified into different types, such as bivalent bispecific antibodies, monovalent bispecific antibodies, and bispecific T-cell engagers. HSAbody is a special type of monovalent bispecific antibody that consists of a single-chain Fv (scFv) and a human serum albumin (HSA) binding domain (HB). HSAbody utilizes the high affinity and long half-life of HSA to improve the stability and efficacy of the drug. HSAbody has the advantages of simplified design, efficient expression, low toxicity, low immunogenicity, etc., but also some limitations and challenges, such as the heterogeneity and variability of HSA, the supply and quality control of HSA, and the competition and saturation of HSA.
Structure and Generation of HSAbody
HSAbody is a monovalent bispecific antibody consisting of a single-chain Fv (scFv) and a human serum albumin (HSA) binding domain (HB). ScFv is a single-chain polypeptide formed by connecting the heavy chain variable region (VH) and the light chain variable region (VL) of an antibody with a flexible linker, with a relatively small molecular weight (about 27 kD) and high penetration. HB is a protein fragment that can bind to HSA with high affinity, usually derived from natural HSA binding proteins such as heparin binding protein, pregnancy-associated plasma protein A, etc. HSAbody forms a non-covalent complex with HSA through HB, thereby utilizing the high abundance (about 600 μM), long half-life (about 19 days), and low immunogenicity of HSA to improve the stability and efficacy of the drug.
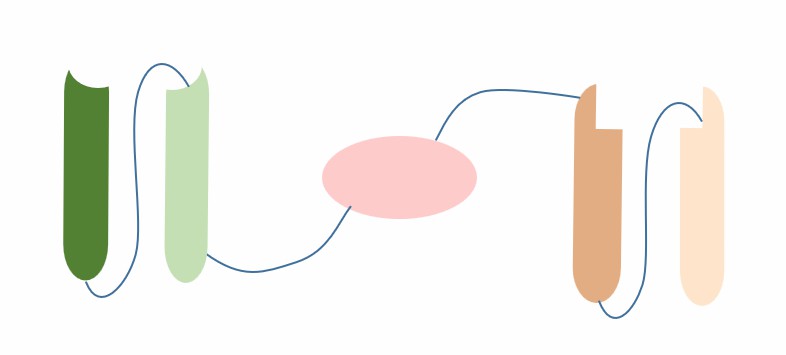
Fig.1 The schematic diagram of the structure of HSAbody (Creative Biolabs)
Clinical Data of HSAbody
HSAbody, a novel type of bispecific antibody that uses human serum albumin (HSA) as a fusion partner, has shown its potential and advantages in cancer therapy. Currently, several HSAbody have been approved by the FDA or are in different stages of clinical trials.
There are two HSAbodies that have been approved by the FDA, namely MM-111 and MFE-CPG2.
Table 1. HSAbodies that have been approved
|
Name
|
Target
|
Approval time
|
Indication
|
Eligible population
|
Country/region
|
|
MM-111
|
HER2XHER4
|
Nov-21
|
HER2-positive advanced breast cancer
|
Patients who have progressed after at least two standard chemotherapy regimens
|
USA
|
|
MFE-CPG2
|
CEA+CPG2
|
Jun-22
|
CEA-positive metastatic colorectal cancer
|
Patients who have progressed after at least one standard chemotherapy regimen
|
USA
|
In addition, there are several HSAbodies that are in different stages of clinical trials, mainly targeting various types of tumors. MFE-23 targets CEA, fused with urokinase (UK), for the treatment of CEA-positive solid tumors. MM-141 targets IGF-1R and HER3 receptors for the treatment of IGF-1R and HER3-positive advanced pancreatic cancer. MFE-25 targets EGFR and CD16 for the treatment of EGFR-positive solid tumors.
Table 2. HSAbodies that are in clinical trials
|
Name
|
Target
|
Stage
|
Indication
|
Number of participants
|
|
MFE-23
|
CEA+UK
|
Phase I/II
|
CEA-positive solid tumors
|
40 people
|
|
MM-141
|
IGF-1RXHER3
|
Phase II
|
IGF-1R and HER3-positive advanced pancreatic cancer
|
88 people
|
|
MFE-25
|
EGFRxCD16
|
Phase I
|
EGFR-positive solid tumors
|
30 people
|
Conclusion
HSAbody is a novel type of bispecific antibody that uses human serum albumin (HSA) as a fusion partner, which can increase the stability and half-life of the antibody while maintaining high affinity and selectivity. HSAbody has been mainly used for cancer therapy, targeting two related tumor markers simultaneously, or delivering cytotoxic drugs or enzymes to tumor cells. Several HSAbodies have been approved by the FDA or are in different stages of clinical trials, showing promising results and advantages over conventional monoclonal antibodies or combination therapies. However, HSAbody also faces some challenges, such as possible dose dependence, nonspecific binding, production cost, and immunogenicity. Therefore, further optimization and improvement of HSAbody are needed to enhance its efficacy and safety. HSAbody represents a new direction and opportunity for the development of antibody-based medicines, and has great potential for the treatment of various diseases in the future.
References
1. Trivedi S, et al. Clinical Pharmacology and Translational Aspects of Bispecific Antibodies. Clin Transl Sci. 2017 Apr;10(2):147-162.
2. Sharma SK, et al. A tumor-targeting pHLIP fusion protein that delivers a novel prodrug of the chemotherapeutic agent monomethyl auristatin E. Mol Cancer Ther. 2014 Mar;13(3):699-709.
3. Kogelberg H, et al. A novel recombinant anti-carcinoembryonic antigen antibody-enzyme fusion protein for antibody-directed enzyme prodrug therapy of colorectal carcinoma. Int J Cancer. 2006 Feb 1;118(3):563-72.
4. Mayer A, et al. Phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours. Br J Cancer. 2006 Sep 4;95(5):596-602.
5. Tolner B, et al. Production of recombinant protein in Pichia pastoris by fermentation. Methods Mol Biol. 2006;308:1-15.
6. Tolner B, et al. Quality control of recombinant antibodies for clinical use: validation of protein production in Pichia pastoris by ELISA and Western blotting. Methods Mol Biol. 2006;308:17-28.
7. Andrady C, et al. Antibody-enzyme fusion proteins for cancer therapy. Immunotherapy. 2011 Feb;3(2):193-211.
8. Marsh D, et al. Stromal features are predictive of disease mortality in oral cancer patients. J Pathol. 2011 Feb;223(4):470-81.
9. Lewis SM, et al. Generation of bispecific IgG antibodies by structure-based design of an orthogonal Fab interface. Nat Biotechnol. 2014 Feb;32(2):191-8.
10. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
11. Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012 Jan-Feb;4(1):182-97.
12. Brinkmann U, Kontermann RE. The making of bispecific antibodies. MAbs. 2017 Jan;9(1):182-212.
13. Labrijn AF, et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019 Aug;18(8):585-608.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










