ImmTAC antibodies are a novel type of bispecific biologics that can activate T cells to kill cancer cells. ImmTAC antibodies consist of two parts: one is an engineered T cell receptor (TCR) that can recognize and bind to human leukocyte antigen (HLA) presented tumor-associated peptides; the other is a single-chain antibody fragment (scFv) that can bind to the CD3 co-receptor on T cells, thereby activating T cells.
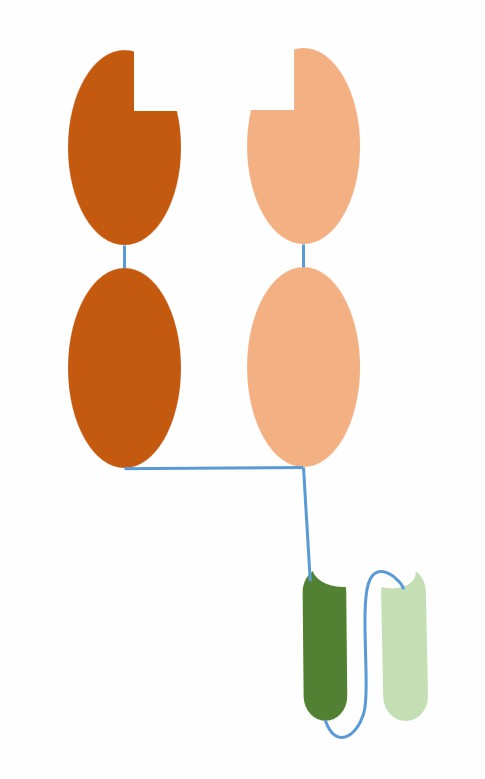
Fig.1 The schematic diagram of the structure of ImmTAC (Creative Biolabs)
The advantage of ImmTAC antibodies is that they can target intracellular and extracellular tumor antigens without being limited by conventional monoclonal antibodies that can only target cell surfaces or secreted proteins. Moreover, they can harness the power of the immune system without relying on exogenous toxic payloads or auxiliary immune cells. The mechanism of action of ImmTAC antibodies is as follows:
ImmTAC antibodies have great potential in tumor immunotherapy, but also face some challenges, such as the complexity of the tumor microenvironment, tumor escape mechanisms, immunogenicity, safety and production difficulty. To overcome these challenges, Immunocore developed ImmTAX platform, which is used to design, screen, and optimize ImmTAC antibodies and collaborate with multiple partners for clinical development and commercialization. Currently, Immunocore has developed several ImmTAC antibodies targeting different tumor types and antigens and achieved some encouraging clinical data.
The Development History of ImmTAC
ImmTAC antibodies are a class of bispecific biologics developed by Immunocore that use engineered T cell receptors (TCRs) to recognize and bind to tumor-associated peptides presented by human leukocyte antigens (HLAs) while using single-chain antibody fragments (scFvs) to bind to the CD3 co-receptor on T cells, thereby activating T cells to kill cancer cells. Immunocore is a biotechnology company headquartered in Oxfordshire, UK, founded in 2008, focusing on developing innovative immunotherapies using TCR technology. The company has its own research and development ImmTAX platform, which is used to design, screen, and optimize ImmTAC antibodies and collaborate with multiple partners for clinical development and commercialization. Currently, Immunocore has developed several ImmTAC antibodies targeting different tumor types and antigens and achieved some encouraging clinical data. The most advanced product is tebentafusp (IMCgp100), an ImmTAC antibody targeting melanoma antigen gp100, for the treatment of metastatic uveal melanoma (mUM). The product completed phase III clinical trial in 2020 and reached the primary endpoint of overall survival (OS).
In addition to tebentafusp, Immunocore also has several other ImmTAC antibodies in different stages of clinical trials, including IMCnyeso-1 (targeting NY-ESO-1), IMC-C103C (targeting MAGE-A4), IMC-F106C (targeting PRAME), etc. These products cover a variety of solid tumors, such as lung cancer, gastric cancer, ovarian cancer, head and neck cancer, etc. Immunocore is also exploring the application of ImmTAC antibodies in infectious and autoimmune diseases, such as chronic hepatitis B, tuberculosis, AIDS, type 1 diabetes, etc.
Clinical Data for ImmTAC
As mentioned above, only one ImmTAC antibody has been approved by the US FDA, namely tebentafusp (IMCgp100), for the treatment of HLA-A*02:01-positive, unresectable, or metastatic uveal melanoma (mUM) adult patients. Tebentafusp is a TCR bispecific protein targeting gp100, consisting of an mTCR specifically binding to the melanocyte antigen gp100 and an anti-CD3 scFv. Tebentafusp showed a significant improvement in overall survival (OS) in a phase 3 clinical trial. Compared with other treatments, the one-year survival rate of the tebentafusp group was 73%, while that of the other treatment group was 58%. Tebentafusp is the first new therapy for metastatic uveal melanoma in 40 years.
Currently, there are several ImmTAC antibodies in different stages of clinical trials, mainly developed by Immunocore or its partners, targeting different tumor-associated antigens, such as NY-ESO-1, MAGE-A4, PRAME, MAGE-A10, AFP, etc. These ImmTAC antibodies are used to treat various solid tumors, such as non-small cell lung cancer, bladder cancer, melanoma, synovial sarcoma, liver cancer, etc. The clinical data for these ImmTAC antibodies are not fully disclosed, but some preliminary results show some efficacy and safety.
Table 1. ImmTAC antibodies in clinical trials
|
Drug name
|
Target
|
Clinical trial phase
|
Partner
|
|
IMCnyeso (GSK3620650)
|
NY-ESO-1 and LAGE-1A
|
Phase 1
|
GSK
|
|
IMC-C103C (GSK3377794)
|
MAGE-A4
|
Phase 1
|
GSK
|
|
IMC-F106C (GSK609227)
|
PRAME
|
Phase 1
|
GSK
|
|
IMC-M114C (GSK609228)
|
MAGE-A10
|
Phase 1
|
GSK
|
|
IMC-A12C
|
AFP
|
Phase 1
|
None
|
References
1. Harper J, et al. An approved in vitro approach to preclinical safety and efficacy evaluation of engineered T cell receptor anti-CD3 bispecific (ImmTAC) molecules. PLoS One. 2018 Oct 15;13(10):e02054911.
2. Zhao Q, et al. Engineered TCR-T Cell Immunotherapy in Anticancer Precision Medicine: Pros and Cons. Front Immunol. 2021 Mar 30;12:6587532.
3. Cole DK, et al. Specificity of bispecific T cell receptors and antibodies targeting peptide-HLA. J Clin Invest. 2019 Nov 1;129(11):4727-47363.
4. Liddy N, et al. Monoclonal TCR-redirected tumor cell killing. Nat Med. 2012 Jun;18(6):980-74.
5. Jakobsen BK, et al. ImmTACs: Novel bi-specific agents for targeted cancer therapy. Oncoimmunology. 2013 Feb 1;2(2):e228915.
6. Boulter JM, et al. Stable, soluble T-cell receptor molecules for crystallization and therapeutics. Protein Eng. 2003 Sep;16(9):707-11.
7. Hassan NJ, et al. T Cell Receptor (TCR)-mimic Antibodies: An Emerging Class of Cancer Immunotherapy Agents. Antibodies (Basel). 2019 Aug 2;8(3):43.
8. Rajasagi M, et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014 Jul 17;124(3):453-62.
9. Johnson LA, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009 Jul 16;114(3):535-46.
10. Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006 Oct 6;314(5796):126-9.
11. Robbins PF, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013 Jun;19(6):747-52.
12. Tran E, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014 May 9;344(6184):641-5.
13. Tran E, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016 Dec 8;375(23):2255-2262.
14. Parkhurst MR, et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol Ther. 2011 Mar;19(3):620-6.
15. Linette GP, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2013 Aug 16;341(6147):641.
16. Cameron BJ, et al. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci Transl Med. 2013 Aug 7;5(197):197ra103.
17. Rapoport AP, et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat Med. 2015 Aug;21(8):914-21.
18. D’Angelo SP, et al. A pilot study of anti-CTLA4 antibody ipilimumab in patients with synovial sarcoma. Sarcoma. 2012;2012:437942.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










