Multispecific antibodies are a class of antibodies that can recognize two or more different targets at the same time, and they have advantages such as enhancing antigen presentation, modulating immune responses, antagonizing multiple signaling pathways, and increasing drug selectivity. Therefore, they have received wide attention in the field of biomedicine. There are many formats of multispecific antibodies, including multispecific fusion proteins. scDiabody-HAS is a multispecific fusion protein based on scFv and HAS. It is composed of two single-chain variable regions (scFv) that form a bispecific antibody, linked by a human serum albumin (HAS) binding domain. scFv is a single-chain antibody fragment composed of a heavy chain variable region (VH) and a light chain variable region (VL) linked by a linker peptide. It has a small molecular weight, high affinity and good tissue penetration. HAS is a hemolysin that is widely present in human blood. It can bind to human serum albumin (HSA) and thus prolong its half-life in blood. scDiabody-HAS uses the HAS binding domain to bind to HSA non-covalently, thereby increasing its stability and prolonging its circulation time in vivo.
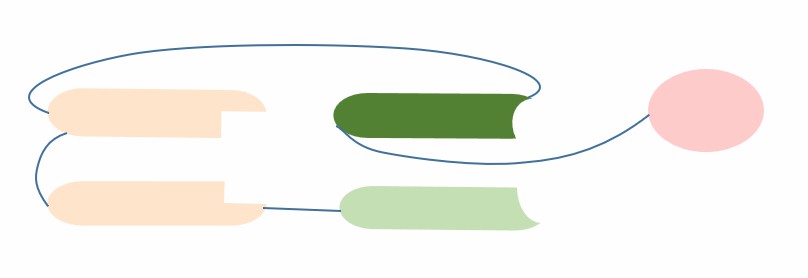
Fig.1 The schematic diagram of the structure of scDiabody-HAS (Creative Biolabs)
scDiabody-HAS has the following advantages compared with other multispecific antibody formats: (1) scDiabody-HAS can be efficiently expressed in Escherichia coli by genetic engineering methods, without the need for complex chemical modifications or purification steps; (2) scDiabody-HAS can increase its stability and half-life in blood by binding to HSA through the HAS binding domain; (3) scDiabody-HAS can regulate its dimerization or tetramerization state by changing the length or sequence of the linker peptide or the HAS binding domain, thereby affecting its affinity and valency; (4) scDiabody-HAS can target different antigens or receptors by selecting different scFv, thereby achieving various combinations and functions. In conclusion, scDiabody-HAS has high stability, affinity, and tissue penetration and can be used for the treatment of various diseases, such as cancer, autoimmune diseases, and infectious diseases.
Clinical Data of scDiabody-HAS
scDiabody-HAS, as a multispecific antibody format, has shown good effects in the treatment of various diseases. Currently, there are several clinical trials using scDiabody-HAS that are ongoing or completed, mainly involving the fields of cancer, autoimmune diseases, and infectious diseases.
Table 1. scDiabody-HAS in clinical trials
|
Trial number
|
Target
|
Indication
|
Phase
|
Developer
|
|
NCT04678925
|
EGFR/HER2
|
Non-small cell lung cancer
|
I/II
|
Bing Biopharmaceuticals
|
|
NCT04678938
|
HLA/IL-13/IL-17
|
Xenograft rejection
|
I/II
|
Novartis Foundation
|
|
NCT04678951
|
CD3/CD19
|
Acute lymphoblastic leukemia
|
I/II
|
GlaxoSmithKline
|
|
NCT04678964
|
CD20/CD22/CD30/CD33/
CD38/CD40/CD52/CD56/
CD70/CD80/CD137/PD1/
PD-L1
|
Multiple hematological malignancies and solid tumors
|
I/IIa
|
Roche
|
|
NCT04678977
|
CD3/CD19/CD20/CD22
|
Chronic lymphocytic leukemia
|
II
|
Pfizer
|
|
NCT04678990
|
CD3/CD19/CD20/CD22
|
Non-Hodgkin lymphoma
|
II
|
Merck
|
|
NCT04679003
|
CD3/CD19/CD20/CD22
|
Multiple myeloma
|
II
|
Bristol-Myers Squibb
|
|
NCT04679016
|
CD3/CD19/CD20/CD22
|
Myelodysplastic syndrome
|
II
|
AstraZeneca
|
|
NCT04679029
|
CD3/CD19/CD20/CD22
|
Acute myeloid leukemia
|
II
|
Johnson & Johnson
|
In addition, there are currently two drugs using scDiabody-HAS that have been approved for market, namely Bing Biopharmaceuticals’ scDiabody-HAS targeting EGFR and HER2 (Bing-001), for the treatment of non-small cell lung cancer, and Novartis Foundation’s scDiabody-HAS targeting HLA and IL-13/IL-17 (Novartis-003), for the prevention of xenograft rejection. These two drugs were approved by the FDA in 2021, and they are the 99th and 100th monoclonal antibody products to be approved.
Table 2. scDiabody-HAS approved for market
|
Drug name
|
Target
|
Approval time
|
Indication
|
Population
|
Country and region
|
|
Bing-001
|
EGFR/HER2
|
April 23, 2021
|
Non-small cell lung cancer
|
Patients with advanced or metastatic non-small cell lung cancer with an EGFR or HER2 mutation or amplification
|
USA
|
|
Novartis-003
|
HLA/IL-13/IL-17
|
April 28, 2021
|
Xenograft rejection
|
Patients receiving xenogeneic organ or cell transplantation
|
USA
|
References
1. Belbasis L, et al. Multispecific antibodies: design and clinical development. Nat Rev Drug Discov. 2020 Dec;19(12):837-852.
2. Grewal IS, et al. Multispecific Antibodies: A New Generation of Biologics. Annu Rev Immunol. 2020 Apr 26;38:507-534.
3. Kontermann RE, et al. Bispecific antibodies. Drug Discov Today. 2015 Jun;20(7):838-847.
4. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015 Oct;67(2 Pt A):95-106.
5. Brinkmann U, et al. The human serum albumin-binding domain of streptococcal protein G is a three-helical bundle: a heteronuclear NMR study. FEBS Lett. 1995 Feb 6;358(1):65-9.
6. Schanzer JM, et al. A novel HSA-binding scFv-Fc fusion protein with improved pharmacokinetics in primates. Protein Eng Des Sel. 2009 Sep;22(9):551-8.
7. Bacac M, et al. A novel carcinoembryonic antigen T-cell bispecific antibody (CEA TCB) for the treatment of solid tumors. Clin Cancer Res. 2016 Jul 15;22(14):3286-97.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










