scFv-KIH is a type of bispecific antibody composed of two single-chain variable fragments (scFvs) connected by knob-into-hole (KIH) technology. scFv is a fusion protein of the variable regions of the heavy (VH) and light chains (VL) of immunoglobulins, linked by a short linker peptide (about 10–25 amino acids), which retains the specificity of the original antibody but lacks the constant regions and the Fc region. KIH technology is a method that uses mutations in the CH3 region of the heavy chain to promote heterodimerization, effectively reducing the formation of homodimers. scFv-KIH has characteristics such as a small molecular weight (about 50–60 kDa), high penetration, and rapid clearance rate, making it suitable for bispecific antibodies that target tumor cells and recruit T cells.
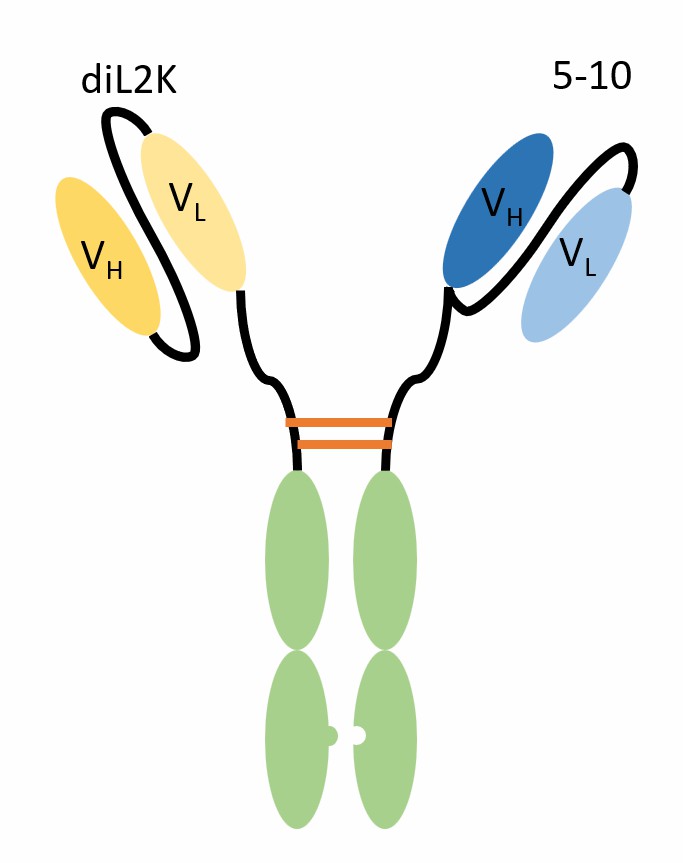
Fig.1 Schematic diagram of scFv-KIH (Creative Biolabs)
Generation Methods of scFv-KIH
scFv-KIH can be generated by various methods, including intracellular expression, extracellular expression, and chemical synthesis. Intracellular expression is the use of transfection or infection to introduce the scFv-KIH gene into host cells, such as CHO cells, HEK293 cells, or hybridoma cells, and then obtain scFv-KIH by culture and purification. This method can take advantage of the cell’s natural folding and modification mechanisms to improve the stability and activity of scFv-KIH, but it also has drawbacks such as low expression efficiency, high purification difficulty, and high cost. Extracellular expression is the use of cell-free protein synthesis systems to transcribe and translate the scFv-KIH gene into protein, and then obtain scFv-KIH by purification. This method can produce scFv-KIH quickly and efficiently, and can flexibly adjust the plasmid ratio between knob and hole, or even add prefabricated knob or hole to increase the yield of heterodimers. But this method may also lead to denaturation or degradation of scFv-KIH, requiring optimization of the conditions and buffer components of the cell-free system. Chemical synthesis is the use of solid-phase peptide synthesis or liquid-phase peptide synthesis to directly synthesize the peptide chains of scFv-KIH, and then obtain scFv-KIH by folding and linking. This method can avoid the use of biological materials such as cells or DNA, reducing the risk of contamination and mutation, and can easily introduce non-natural amino acids or other modifications to enhance the function of scFv-KIH. But this method also has problems such as low synthesis efficiency, low yield, and high cost.
Clinical Data of scFv-KIH
scFv-KIH is a novel type of bispecific antibody that currently targets mainly CD3, CD19, CD20, CD33, EpCAM, HER2, etc. in clinical trials led by companies or research institutions such as Amgen, Roche, etc. scFv-KIH has shown good safety and efficacy in clinical trials, with significant therapeutic effects on various hematological malignancies and solid tumors. Some scFv-KIHs have entered phase III clinical trials or applied for accelerated approval. Currently, two scFv-KIH products have been approved, namely Blinatumomab (Blincyto\u0001) developed by Amgen and Emicizumab (Hemlibra\u0001) developed by Roche.
Table 1. Overview of scFv-KIH products on the market
|
scFv-KIH
|
Target
|
Approval date
|
Indication
|
Population
|
Country/Region
|
|
Blinatumomab
|
CD3/CD19
|
Dec-14
|
Relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL)
|
Adults and children
|
USA
|
|
|
|
Nov-15
|
|
|
EU
|
|
|
|
Sep-16
|
Very rare Philadelphia chromosome-positive (Ph+) B-ALL patients
|
Adults and children
|
USA
|
|
|
|
Jul-17
|
Minimal residual disease-positive (MRD+) B-ALL patients
|
Adults and children
|
USA
|
|
|
|
Mar-18
|
|
|
EU
|
|
Emicizumab
|
Factor IXa/ Factor X
|
Nov-17
|
Prevention of bleeding episodes in hemophilia A patients, regardless of the presence of factor VIII inhibitors
|
Adults and children (≥12 years old)
|
USA
|
|
|
|
Feb-18
|
Prevention of bleeding episodes in hemophilia A patients, regardless of the presence of factor VIII inhibitors, or prevention or treatment of bleeding episodes in patients who cannot receive or respond poorly to factor VIII treatment.
|
Adults and children (≥12 years old)
|
EU
|
|
|
|
Oct-18
|
Prevention of bleeding episodes in hemophilia A patients, regardless of the presence of factor VIII inhibitors.
|
Children (<12 years old)
|
USA
|
References
1. Spiess C, et al. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol. 2015;67(2 Pt A):95–106.
2. Bradbury AR, et al. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol. 2011;29(3):245–54.
3. Monnier PP, et al. Bispecific antibodies for delivery of therapeutics to the CNS. MAbs. 2013;5(3):298–305.
4. Sandomenico A, et al. Generation and characterization of monoclonal antibodies against a cyclic variant of Hepatitis C Virus E2 epitope 412-422. J Virol. 2016;90(7):3745–59.
5. Ahmed AR, et al. Treatment of pemphigus vulgaris with rituximab and intravenous immune globulin. N Engl J Med. 2006;355(17):1772–9.
6. Bates N, et al. Current challenges and opportunities in the development of bispecific antibodies for cancer immunotherapy. Antibodies (Basel). 2019;8(4):43.
7. Liu H, et al. Fc engineering for developing therapeutic bispecific antibodies and novel scaffolds. Front Immunol. 2017;8:38.
8. Kontermann RE. Dual targeting strategies with bispecific antibodies. MAbs. 2012;4(2):182–97.
9. Baeuerle PA, et al. Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res. 2009;69(12):4941–4.
10. Chames P, et al. Bispecific antibodies for cancer therapy: the light at the end of the tunnel? MAbs. 2009;1(6):539–47.
11. Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol. 2011;22(6):868–76.
12. Labrijn AF, et al. Efficient generation of stable bispecific IgG1 by controlled Fab-arm exchange. Proc Natl Acad Sci U S A. 2013;110(13):5145–50.
13. Brinkmann U, et al. The making of bispecific antibodies. MAbs. 2017;9(2):182–212.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










