In some species, there is a type of antibody that does not require light chains, called heavy chain-only antibodies (HCAbs). HCAbs are composed of only heavy chains, and their heavy chain variable regions (VH) can form a complete antigen-binding site by themselves. HCAbs were first discovered in camelids, and later in cartilaginous fish. HCAbs have some unique advantages, such as small molecular weight, high affinity, high stability, high solubility, etc., making them ideal candidates for biologics. In order to develop humanized HCAbs, some transgenic animal models have been established, such as transgenic camels, transgenic mice and transgenic rats. These animals can produce HCAbs with humanized VH regions after immunization. By using phage display or other techniques, HCAbs with high specificity and affinity can be screened from these animals. In addition to being used as separate biologics, HCAbs can also be used as basic units to construct multispecific antibodies. Multispecific antibodies are a class of antibodies that can simultaneously recognize two or more different antigens or epitopes, with a wider range of applications. By connecting two or more different VH regions, HCAbs with bispecificity or multispecificity can be prepared. This type of HCAb is called tetravalent HCAb because it has four antigen-binding sites. Tetravalent HCAb has some advantages that conventional bispecific antibodies do not have, such as simple design, efficient expression, good biological activity, etc. Tetravalent HCAb has been used to neutralize some bacterial toxins or viruses, showing good results.
Structure and Preparation of Tetravalent HCAb
Tetravalent HCAb is a type of bispecific antibody composed of four VH regions, each connected by a humanized heavy chain constant region (hCH), forming a bivalent heavy chain unit. Two bivalent heavy chain units form a tetravalent complex through the interaction between hCH regions, having four antigen-binding sites. As shown in Figure 1, Tetravalent HCAb can simultaneously recognize two different antigens or epitopes, such as A and B, thus achieving bispecificity.
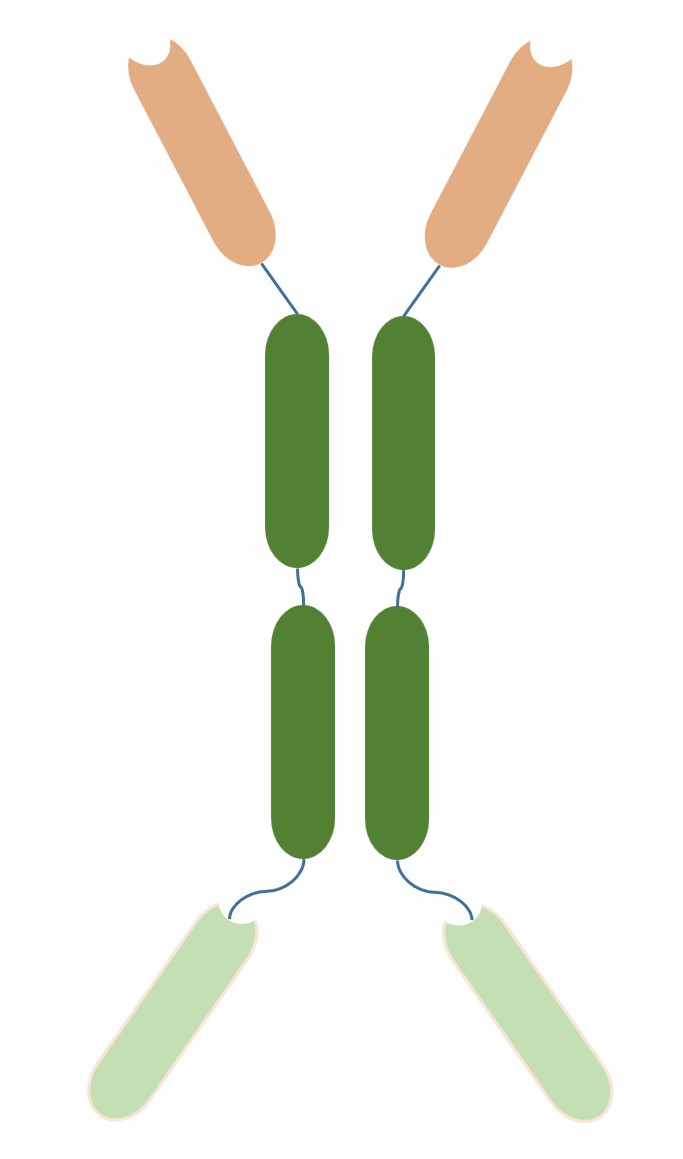
Fig.1 Schematic diagram of tetravalent HCAb (Creative Biolabs)
The preparation method for tetravalent HCAb mainly includes the following steps:
-
First, using transgenic animal models, such as transgenic camels, transgenic mice or transgenic rats, immunize the target antigen or epitope, thereby inducing the production of HCAbs with humanized VH regions. These animals have a large number of humanized heavy chain V(D)J gene arrangements, which can produce diversified and high-affinity HCAbs.
-
Second, using phage display or other techniques, screen HCAbs with high specificity and affinity from immunized animals. For different antigens or epitopes, different HCAbs can be screened to construct bispecific antibodies.
-
Third, using gene recombination technology, connect two or more different VH regions, and insert a DNA fragment encoding a humanized hCH region (without CH1) between every two VH regions. In this way, a bivalent heavy chain unit can be constructed, containing two different VH regions and one hCH region.
-
Fourth, using expression systems, such as mammalian cells, yeast cells or insect cells, express the bivalent heavy chain unit and obtain the target protein by purification techniques. Due to the interaction ability between hCH regions, two bivalent heavy chain units can spontaneously form a tetravalent complex, that is, tetravalent HCAb.
Clinical Data of Tetravalent HCAb
Tetravalent HCAb, as a novel type of bispecific antibody, has high neutralizing activity and therapeutic potential and has been used for clinical trials or applications against some bacterial toxins or viruses. This part will introduce the relevant information about tetravalent HCAbs that have been approved for marketing and are in clinical trials.
So far, there is one tetravalent HCAb that has been approved for marketing, namely tetravalent HCAb neutralizing Staphylococcus aureus leukotoxins (PVL). PVL is a bicomponent pore-forming toxin composed of two components (LukS-PV and LukF-PV), which can form pores on the host defense cells, leading to osmotic lysis and cell death. PVL is associated with outbreaks of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and directly involved in the pathogenesis of Staphylococcus aureus-related diseases, such as pyogenic necrotizing skin infections, osteomyelitis, bacteremia, purpura fulminans, and necrotizing pneumonia. A humanized tetravalent HCAb generated from transgenic mice immunized with PVL was developed, which can effectively neutralize the activity of PVL. The tetravalent HCAb consists of one anti-LukS-PV VH region and one anti-LukF-PV VH region, each connected by a humanized hCH region. The tetravalent HCAb can bind to both LukS-PV and LukF-PV and prevent them from binding to the cell surface receptors and pore formation. The tetravalent HCAb can also bind to γ-hemolysin C (HlgC) and inhibit the pore formation of HlgC and HlgB. The tetravalent HCAb was approved by the European Medicines Agency (EMA) in 2011 for the treatment of severe eye infections caused by PVL-positive Staphylococcus aureus, such as corneal ulcers, endophthalmitis, etc. The trade name of the tetravalent HCAb is LukAb, developed and produced by Innate Pharma in France.
Currently, there are some tetravalent HCAbs that are in different stages of clinical trials, mainly for neutralizing some viruses, such as influenza virus, HIV virus, and Ebola virus.
Table 1. Tetravalent HCAbs in clinical trials
|
Target
|
Registration number
|
Trial phase
|
Sample size
|
Led by which companies/institutions
|
|
Influenza virus (HA1 and HA2)
|
NCT04396064
|
I/IIa
|
60 healthy volunteers and 60 influenza patients
|
Teneobio, Inc. and Abbvie Inc.
|
|
HIV (gp120 and gp41)
|
-
|
-
|
-
|
Erasmus Medical Center and Harbour Antibodies BV
|
|
Ebola virus (GP1 and GP2)
|
-
|
-
|
-
|
Erasmus Medical Center and Harbour Antibodies BV
|
References
1. Laventie BJ, et al. Heavy chain-only antibodies and tetravalent bispecific antibody neutralizing Staphylococcus aureus leukotoxins. Proc Natl Acad Sci U S A. 2011 Sep 27;108(39):16404-9.
2. Chen T, et al. Generation of a tetravalent bispecific antibody with high anti-influenza virus activity using a novel single-chain variable fragment assembly method. J Virol. 2015 Dec;89(23):12002-13.
3. Hamers-Casterman C, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993 Jun 3;363(6428):446-8.
4. De Genst E, et al. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci U S A. 2006 Mar 14;103(11):4586-91.
5. Harmsen MM, et al. Llama heavy-chain V regions consist of at least four distinct subfamilies revealing novel sequence features. Mol Immunol. 2000 Aug-Sep;37(10):579-90.
6. De Meyer T, et al. Generation and characterization of small single domain antibodies inhibiting human tumor necrosis factor receptor 1. J Biol Chem. 2014 Jul 18;289(29):20238-51.
7. De Haard HJ, et al. Llama antibodies against a lactococcal protein located at the tip of the phage tail prevent phage infection and development of phage resistance in bacteria. Appl Environ Microbiol. 2005 Jan;71(1):138-44.
8. Van Rompaey D, et al. Generation of a tetravalent anti-CD20/CD3 bispecific antibody for the treatment of B cell malignancies. MAbs. 2017 Oct;9(7):1140-53.
9. Wang Q, et al. Design and Production of Bispecific Antibodies. Antibodies (Basel). 2019 Aug 2;8(3):43.
Our products and services are for research use only, and not for use in diagnostic or therapeutic procedures.
Welcome! For price inquiries, we will get back to you as soon as possible.
To order, please email
INQUIRY










